Search
Search
Clinical trial will evaluate new therapy for patients with treatment-resistant depression as a result of bipolar disorder
LONDON, ON – Researchers at Lawson Health Research Institute are offering new hope to patients with treatment-resistant depression through participation in a national clinical trial. The study is the first randomized controlled trial to examine the efficacy of a new treatment called magnetic seizure therapy (MST) for patients with treatment-resistant depression as a result of bipolar disorder.
Treatment-resistant depression is a severe form of depression that does not respond to traditional therapies like medication. It is particularly common in patients with depression from bipolar disorder who are left with limited treatment options.
For years electroconvulsive therapy (ECT) has been the gold standard for patients with treatment-resistant depression. ECT uses an electric field to induce a seizure that provides a therapeutic benefit. But while ECT is effective, many patients opt out of treatment due to stigma surrounding the therapy and the potential for cognitive side effects like disorientation and amnesia.
MST has emerged as a promising alternative. MST works in a similar way to ECT but uses a focused magnetic field as opposed to electricity. As a result, MST induces a more focused seizure to reduce the risk of cognitive side effects.
“Magnetic seizure therapy has already been shown as a promising treatment for major depressive disorder or unipolar depression,” explains Dr. Amer Burhan, local site lead, researcher at Lawson and neuropsychiatrist at St. Joseph’s Health Care London. “For the first time, we’re studying how effective the treatment is for depression as a result of bipolar disorder and whether it can reduce the risk of cognitive side effects associated with electroconvulsive therapy.”
The clinical trial is being led by the Centre for Addiction and Mental Health (CAMH) and will also be offered through Lawson and University of British Columbia (UBC) Hospital. Lawson researchers will invite eligible patients with treatment-resistant depression from bipolar disorder to participate in the trial at Parkwood Institute, a part of St. Joseph’s Health Care London.
Eligible patients will be randomized to receive either ECT or MST. Patients will be offered support throughout the study with the goal of improving patient outcomes in both groups. Patient outcomes will be compared to study the efficacy of MST and whether it is associated with reduced cognitive side effects.
MST will be delivered under anesthesia in 12 to 20 sessions. Sessions will last 10 to 15 minutes each with 60 to 90 minutes of recovery time.
“Magnetic seizure therapy holds promise of one day replacing electroconvulsive therapy as the gold standard for treatment-resistant depression,” says Dr. Burhan. If proven as a viable first-line treatment, MST would be very easy to implement in existing ECT clinics. MST would therefore be readily available to patients in need.
“We are on the leading edge of the field of brain stimulation for treatment-resistant depression in collaboration with CAMH and UBC,” says Dr. Burhan. “Our goal is to continue informing the care process through clinically-relevant research that serves patients, medical professionals and the public.”
-30-
Lawson Health Research Institute is one of Canada’s top hospital-based research institutes, tackling the most pressing challenges in health care. As the research institute of London Health Sciences Centre and St. Joseph’s Health Care London, our innovation happens where care is delivered. Lawson research teams are at the leading-edge of science with the goal of improving health and the delivery of care for patients. Working in partnership with Western University, our researchers are encouraged to pursue their curiosity, collaborate often and share their discoveries widely. Research conducted through Lawson makes a difference in the lives of patients, families and communities around the world. To learn more, visit www.lawsonresearch.ca.
Senior Media Relations Consultant
Communications & Public Engagement
T: 519-685-8500 ext. 73502
Celine.zadorsky@lhsc.on.ca
Clinical trial will evaluate new therapy for treatment-resistant depression as a result of bipolar disorder
Treatment-resistant depression is a severe form of illness that does not respond to traditional therapies like medication and counselling. It is particularly common in patients with depression from bipolar disorder who are left with limited treatment options.
“There are some mental illnesses that can become resistant to therapy, similar to how infections, for example, can become resistant to antibiotics,” explains Dr. Amer Burhan, researcher at Lawson Health Research Institute and neuropsychiatrist at St. Joseph’s Health Care London. “Patients with those illnesses need more options.”
Brain stimulation is a field that holds promise for this patient population.
“When people are in a state of depression, research shows their brain networks are not functioning properly,” says Dr. Burhan. “Brain stimulation aims to stimulate neurons in the brain to correct activity and improve patient outcomes.”
Through involvement in a national clinical trial, Dr. Burhan and his research team at Lawson are offering new hope with a treatment called magnetic seizure therapy (MST). The study is the first randomized controlled trial to examine the efficacy of MST for patients with treatment-resistant depression as a result of bipolar disorder.
For years electroconvulsive therapy (ECT), one form of brain stimulation, has been the gold standard for patients with treatment-resistant depression. ECT uses an electric field to induce a seizure that provides a therapeutic benefit by stimulating the brain. But while ECT is effective, many patients opt out of treatment due to stigma surrounding the therapy and its potential for cognitive side effects like disorientation and amnesia.
MST has emerged as a promising alternative. MST works in a similar way to ECT but uses a focused magnetic field as opposed to electricity. As a result, MST induces a more focused seizure to reduce the risk of cognitive side effects.
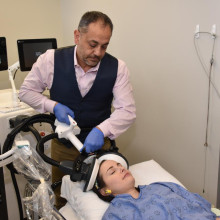
“Magnetic seizure therapy has already been shown as a promising treatment for major depressive disorder or unipolar depression,” explains Dr. Amer Burhan, local site lead for the clinical trial. “For the first time, we’re studying how effective the treatment is for depression as a result of bipolar disorder and whether it can reduce the risk of cognitive side effects associated with electroconvulsive therapy.”
The clinical trial is being led by the Centre for Addiction and Mental Health (CAMH) and will also be offered through Lawson and University of British Columbia (UBC) Hospital. Lawson researchers will invite approximately 30 eligible patients with treatment-resistant depression from bipolar disorder to participate in the trial at Parkwood Institute, a part of St. Joseph’s Health Care London.
Eligible patients will be randomized to receive either ECT or MST. Patients will be offered support throughout the study with the goal of improving patient outcomes in both groups. Patient outcomes will be compared to study the whether MST is effective and whether it is associated with reduced cognitive side effects.
MST will be delivered under anesthesia in 12 to 20 sessions. Sessions will last 10 to 15 minutes each with 60 to 90 minutes of recovery time.
“Magnetic seizure therapy holds promise of one day replacing electroconvulsive therapy as the gold standard for treatment-resistant depression, but we need to learn more about where it fits in our toolbox of potential treatments,” says Dr. Burhan.
If proven as a viable first-line treatment, MST would be very easy to implement in existing ECT clinics. MST would therefore be readily available to patients in need.
“We are on the leading edge of the field of brain stimulation for treatment-resistant depression in collaboration with CAMH and UBC,” says Dr. Burhan. “Our goal is to continue informing the care process through clinically-relevant research that serves patients, medical professionals and the public.”
Those who would like more information about the trial can email @email or @email.
Clinical use of EpiSign proven for diagnosing rare heritable disorders
LONDON, ON – A study led by researchers at Lawson Health Research Institute (Lawson) provides clinical validation of EpiSign, a molecular genomics test that diagnoses rare, heritable neurodevelopmental conditions.
Invented at Lawson by Dr. Bekim Sadikovic, the diagnostic test uses machine learning to analyze the EpiSign Knowledge Database. This database compiles information on rare genetic diseases using laboratory analyses of the epigenome from patients with suspected genetic abnormalities. The epigenome is a process that can change the expression of a gene without changing the gene sequence.
“Using 211 blood samples, we measured test performance and diagnostic yield in 207 subjects from two different cohorts,” explains Dr. Sadikovic, lead researcher at Lawson and Scientific and Clinical Director of the Verspeeten Clinical Genome Centre at London Health Sciences Centre (LHSC). The targeted cohort were subjects with previous genetic findings that were ambiguous or inconclusive. The screening cohort were those with clinical findings consistent with hereditary neurodevelopment syndromes but with no previous genetic findings.
“Of the 207 subjects tested, 57 were positive for a diagnostic episignature including 48 in the targeted cohort, and 8 in the screening cohort. Only four remained inconclusive after EpiSign analysis,” says Dr. Sadikovic. “This gives us strong evidence for the clinical use of EpiSign, as well as the ability to provide conclusive findings in the majority of subjects tested.”
While currently there are limited treatment options associated with many of these conditions,
providing a diagnosis can help physicians better predict the course of the disease, and allows for better planning and support for the patient. EpiSign is the only test in the world that has been clinically validated for testing these kinds of genetic disorders.
“Patients with rare diseases often wait years and undergo numerous exams and tests before receiving a correct diagnosis, if one is found at all,” says Matthew Tedder, PhD, staff scientist at the Greenwood Genetic Center, one of the EpiSign clinical testing laboratories. “EpiSign provides an additional high-yield diagnostic tool for clinicians to include in their evaluation of patients with undiagnosed diseases, providing better medical management for patients and hope for their families.”
The study, “Clinical epigenomics: genome-wide DNA methylation analysis for the diagnosis of Mendelian disorders", is published in February’s Genetics in Medicine and was completed in collaboration with the Greenwood Genetic Center and the University of Amsterdam.
-30-
Lawson Health Research Institute is one of Canada’s top hospital-based research institutes, tackling the most pressing challenges in health care. As the research institute of London Health Sciences Centre and St. Joseph’s Health Care London, our innovation happens where care is delivered. Lawson research teams are at the leading-edge of science with the goal of improving health and the delivery of care for patients. Working in partnership with Western University, our researchers are encouraged to pursue their curiosity, collaborate often and share their discoveries widely. Research conducted through Lawson makes a difference in the lives of patients, families and communities around the world. To learn more, visit www.lawsonresearch.ca.
Senior Media Relations Consultant
Communications & Public Engagement
T: 519-685-8500 ext. 73502
Celine.zadorsky@lhsc.on.ca