Search
Search
National award honours innovator Frank Prato’s excellence in medical physics
Dr. Frank Prato is a man of many firsts:
First in Canada to conduct magnetic resonance brain imaging. A pioneer in magnetic resonance cardiac imaging. Driving force behind the installation of Canada’s first PET/MR scanner. Founder of the Canadian Organization of Medical Physicists (COMP) while president of the Canadian College of Medical Physicists.
And while Dr. Prato admits to a competitive streak that constantly propels him to break new ground in medical physics, the renowned, prolific researcher is keen to note these innovations have been part of a larger team effort.
“My career has been filled with opportunities to work with and train some spectacular scientists who have made major contributions across Canada and around the world,” he says. “I’m proud of the whole group that has developed over the years and the support St. Joseph’s has provided.”
Chief Medical Physicist at St. Joseph’s Health Care London (St. Joseph’s), Dr. Prato has been awarded the 2024 COMP Gold Medal, the organization’s highest award for outstanding career achievement.
“Dr. Prato’s ground-breaking work in the field of medical physics has not only advanced the scientific community but also significantly impacted patient care in Canada and beyond,” says COMP President Boyd McCurdy, “His pursuit of innovation and excellence exemplifies the highest standards of our profession, and we celebrate his outstanding contributions to medical physics with admiration and gratitude.”

Dr. Prato is also Assistant Scientific Director and Imaging Program Leader at Lawson Health Research Institute (Lawson) and professor of medical imaging and medical biophysics at Western University.
“I have worked with Frank for 36 years now and have come to recognize him as one of the finest people I know,” says Dr. Ting-Yim Lee, Director of PET/CT Research at Lawson, medical physicist at St. Joseph’s Hospital, and one of the nominators of Dr. Prato for the award.
“Frank is unfailingly helpful and authentic, a great listener and a tenacious problem-solver. He demonstrates excellence and professionalism in medical physics locally, nationally and internationally.”
Dr. Lee cites Dr. Prato’s leadership in being “at the forefront of numerous international innovations in nuclear medicine and diagnostic radiology.”
Throughout his 48 years as a medical physicist, Dr. Prato has been inspired by the potential of technology’s reach into human health.
“I’ve always wanted to work in an area where we can do research, with a vision of what’s going to be important in patient health. I get excited about being on the leading edge of discovery that’s embedded in patient care.”
Critical advancements in nuclear medicine and diagnostic radiology, thanks to the work of Dr. Prato and his St. Joseph’s/Lawson team, have included:
- Introducing the first bone mineral density imaging procedure on a patient in Canada, a tool now essential for managing osteoporosis.
- Performing the first magnetic resonance brain imaging in Canada, setting a national standard.
- Pioneering magnetic resonance cardiac imaging techniques, enhancing the understanding of myocardial scarring and blood flow assessment.
- Introducing the first PET/CT and PET/MR scanners in Canada, revolutionizing molecular imaging and proving the economic value of advanced imaging technologies.
- Imaging the brains of premature infants, a world first.
- Developing Canada’s first self-sustaining cyclotron infrastructure.
- Conducting the world’s first MRI-compatible, high-resolution brain PET scan.
- Early diagnosis and treatment of dementia, mental illness and prostate cancer.
Dr. Prato’s leadership extends beyond his technical achievements. As the founder of COMP, an organization that now includes more than 800 professionals, he played a crucial role in establishing the organization, advocating for medical physicists' independent voice and professional growth.
His tenure as President and board member of the Canadian College of Physicists in Medicine (CCPM) was marked by significant advancements, including enhancing certification processes and establishing reciprocity with the American Board of Medical Physics. Dr. Prato also received the Valuable Service Award from CCPM in 2002 and was named a Fellow of COMP in 2013.
Earlier this spring, Dr. Prato received a Dean’s Award of Excellence for Research Faculty from Western University’s Schulich School of Medicine & Dentistry. He is also the sole Canadian to have won the d'Arsonval Award, an international honour from the Bioelectromagnetics Society.
A dedicated mentor, Dr. Prato has guided more than 60 Masters of Science students, PhD students and Post-Doctoral Fellows, many of whom have won awards and secured prominent positions in the field. His mentorship has been instrumental in the success of numerous scientists within the Imaging Program at Lawson, contributing to a legacy of innovation for years to come.
“At 78 years old,” he says, “I am pleased to say I have achieved things at St. Joseph’s that will far outlast me.”
National research collaboration leads to Health Canada approval of life-saving radioisotope production
A Canadian consortium, which includes Lawson Health Research Institute (Lawson), TRIUMF, BC Cancer and Centre for Probe Development and Commercialization, is the first in the world to receive regulatory approval to produce the world’s most commonly used medical isotope, technetium-99m (Tc-99m), using small particle accelerators known as cyclotrons.
Tc-99m is used in tens of millions of nuclear medicine procedures globally each year. These include cancer scans, cardiac tests, as well as several other diagnostic procedures. As the world moves away from uranium-based nuclear reactors, there has been growing concern in the medical community of a global shortage of these life-saving compounds. This development helps secure a domestic supply of Tc-99m for Canadian patients.
For over a decade, Dr. Michael Kovacs, Director, Lawson Cyclotron & PET Radiochemistry Facility, and Steven Foster, Business Manager, Lawson Imaging, have been working on research that has contributed significantly to this major development. They have demonstrated the successful production of Tc-99m on a standard hospital-based cyclotron at Lawson, confirming that this technology can be used by almost half of the world’s already installed cyclotrons. Clinical trials were conducted across Canada and locally at St. Joseph’s Health Care London.
“In 2011, we received federal funding to see if we could develop a technology to produce Tc-99m in hospital cyclotrons,” explains Dr. Kovacs. “Canada’s Chalk River nuclear reactor was one of the world’s largest suppliers, and it was set to close in 2016. Cyclotron facilities offer a greener, safer, more sustainable approach for producing critical medical isotopes. Our goal was to find an alternative to the traditional means of producing this isotope, and we have been successful.”
Nuclear medicine is a functional imaging technique, meaning that it images biological function. Medical isotopes are converted to radiopharmaceuticals which get injected into the patient during a procedure. According to the specific biological properties of the isotope, they move throughout the body, rendering a 3D map of where the isotope has gone. This gives researchers and medical professionals valuable information of how various physiological processes are performing.
“Canada is a global leader in nuclear imaging technology. With the help of our collaborators across the country, we have home-grown technology to produce commercial quantities of Tc-99m on common cyclotrons,” adds Mr. Foster. “This technology has been patented and licensed to ARTMS Inc., a spin-off from the consortium, and is now being commercialized and sold throughout the world.”
The process was approved by Health Canada in November, 2020, and is expected to be deployed in British Columbia by 2022.
New Alzheimer’s research aims to improve treatment and support for patients with agitation
Two new interventional studies have been brought to London, focused on improving quality of life for patients with Alzheimer’s disease and their caregivers. Both hope to improve upon standard approaches to treating agitation, a core symptom of Alzheimer’s.
Agitation is a significant source of stress for patients and caregivers. It is complex and difficult to treat. Often, families do not know about this particular symptom of Alzheimer’s and are not properly trained on how to manage care while dealing with agitation.
“These studies are designed to have a direct impact on patients, families and care providers, to improve quality of life and function in those suffering from agitation due to Alzheimer’s,” says Dr. Amer Burhan, Associate Scientist at Lawson Health Research Institute (Lawson) and Geriatric Neuropsychiatrist, St. Joseph’s Health Care London (St. Joseph’s).
Parkwood Institute, a part of St. Joseph’s, is one of multiple sites participating in these studies across Canada and the United States.
One study aims to identify patients early in their diagnosis, while they are living at home or in the community, and apply a comprehensive psychosocial approach, with or without medication, to help with the management of agitation. “We hope to identify participants and have them participating in our program before they experience a crisis due to agitation,” explains Dr. Burhan.
Psychosocial intervention is a way of helping patients and caregivers understand the reasons for agitation. Agitation can develop due to a wide range of causes. For example, patients may just be bored and need help to find something meaningful to occupy their time, they could be upset about something in their current environment, or may be suffering from physical discomfort or pain.
Interventions can include communicating with patients in a manner that creates calm, scheduling meaningful activities, and maintaining routine and rhythm in life. The research team will connect with families early after diagnosis to give them the tools and support they need.
Initially, participants will be treated using structured psychosocial intervention to help reduce and manage their agitation. After three weeks, they will be reassessed and if significant agitation continues to persist, the patient will be randomly selected to receive either a placebo, or medication known as S-Citalopram to treat agitation while they continue to receive psychosocial care.
Sylvia Wilson is the wife of one of the study participants. By enrolling in this trial, she feels she has gained a much better understanding of her husband’s disease, and is grateful for the support that study participants receive.
“My husband typically does not like going to visit doctors, but Dr. Burhan and his team are great,” says Wilson. “They understand agitation, and other symptoms of the disease very well, and I notice a difference in his mood with the treatment he receives through the study.”
Participants are still able to receive care from their primary physician and care teams, with the study providing an added layer of support.
Another study is focused on Alzheimer’s patients who are admitted to hospital or living in long-term care. The aim is to standardize the approach to care for agitation related to Alzheimer’s. After baseline assessment, participants will be randomized to receive the current treatment as per usual, or an integrated care pathway derived from evidence-informed treatment guidelines. These include washing out medications that have not helped, adding individualized behavioral and environmental support, and if medications are needed, use a specific set of medications and dosages based on best evidence.
“Better understanding agitation is a growing area of interest in geriatric research. The work being done locally is part of an international effort to create a paradigm shift in treating patients with Alzheimer’s disease and agitation,” explains Dr. Burhan.
Researchers are ready to offer these studies to patients and their families, hoping to make these treatment protocols an integral part of care for patients with agitation due to Alzheimer’s disease. Those interested in learning more about these studies can contact Dr. Burhan at @email or call 519-646-6100 x. 48170.
In the media: Study on 'agitation' in Alzheimer's patients seeks participants
New clinical protocol after general surgery cuts opioid prescribing in half
In recent years deaths from opioid overdoses have become one of the most common injury-related deaths in North America. The continent also has the highest per capita rate of opioid prescription in the world.
Recognizing the role that opioid prescribing plays in the national opioid crisis, a team of researchers at Lawson Health Research Institute and Western University have developed a new clinical protocol called STOP Narcotics. A study demonstrating the efficacy of their protocol was presented at the American College of Surgeons Clinical Congress in Boston, Massachusetts on October 24.
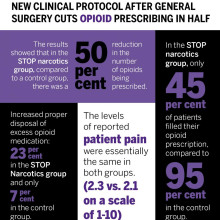
The protocol includes a combination of patient and health care provider education and an emphasis on non-opioid pain control. The study found that they were able to reduce the overall amount of opioids being prescribed after general surgery by 50 per cent while still adequately treating a patient’s post-operative pain.
“By significantly reducing the amount of opioids prescribed, this decreases the exposure risk and potential for misuse of narcotic medication,” said Dr. Luke Hartford, a resident in general surgery at Western’s Schulich School of Medicine & Dentistry and first author on the study. “This also decreases excess medication available to be diverted to individuals for whom it was not intended.”
The study involved 416 patients at London Health Sciences Centre (LHSC) and St. Joseph’s Health Care London who underwent laparoscopic cholecystectomy or open hernia repair. They received medication for post-operative pain through the standardized protocol, specifically acetaminophen (Tylenol) and a non-steroidal anti-inflammatory drug (Naproxen) for the first 72 hours post-surgery.
The protocol instructs physicians to write a limited prescription of ten pills of opioids (Tramadol), with an expiry date of seven days after surgery, with instructions for the patient to fill this prescription only if adequate pain control was not otherwise achieved. There are also instructions on proper disposal of unused medication for the patient.
Dr. Ken Leslie, scientist at Lawson, associate professor in the Department of Surgery at Schulich Medicine & Dentistry, and Chair/Chief of the Division of General Surgery at London Health Sciences Centre led the implementation of the new protocol.
“We recognized that before STOP Narcotics, every surgeon had a different approach to pain control, and that most surgeons were prescribing more narcotics than are actually needed,” said Dr. Leslie. “When we looked at the data from this new protocol, we saw that the patient’s pain-control was just as good with this pathway, without a huge prescription for narcotics.”
The results showed that in the STOP narcotics group, compared to a control group, there was a 50 per cent reduction in the number of opioids being prescribed. They also demonstrated that only 45 per cent of patients actually filled their opioid prescription, compared to 95 per cent in the control group, and they were also able to increase appropriate disposal of excess opioid medication from 7 per cent in the control group to 23 per cent in the STOP Narcotics group. The levels of reported post-operative pain were the same in both groups.
The group now hopes to expand the protocol for applications beyond general surgery.
“If we can decrease the opioid exposure risk in our patients, and decrease the amount of excess medication available for diversion, and spread this to other institutions and surgical procedures and specialties, this has the potential to significantly impact the opioid crisis,” said Dr. Patrick Murphy, a resident in general surgery at Schulich Medicine & Dentistry and co-author on the study.
The study, “The Standardization of Outpatient Procedure (STOP) Narcotics: A Prospective Noninferiority Study to Reduce Opioid Use in Outpatient General Surgical Procedures,” is published in the Journal of the American College of Surgeons.
| Dr. Ken Leslie | Dr. Luke Hartford | Dr. Patrick Murphy |
Image

|
Image

|
Image

|
New device could reduce COVID-19 infection risk and demand for invasive ventilators
LONDON, ON – Led by Lawson Health Research Institute, London Health Sciences Centre (LHSC), University Health Network (UHN) and General Dynamics Land Systems-Canada (GDLS-Canada), researchers have designed a non-invasive ventilation mask that could significantly reduce aerosolization – the production of airborne respiratory droplets that may contain viruses or bacteria – when treating patients with COVID-19. The new device aims to reduce infection risks associated with non-invasive ventilation and lessen the demand for invasive ventilators. It is currently being tested through a clinical trial with patients at LHSC.
“Since the beginning of this pandemic, there have been global concerns about a shortage of ventilators,” says Dr. Tarek Loubani, Lawson Associate Scientist and Emergency Department Physician at LHSC. “Non-invasive ventilators like CPAP (continuous positive airway pressure) and BiPAP (bi-level positive airway pressure) machines are associated with an increased risk of COVID-19 transmission and so many hospitals have moved directly to invasive ventilation.”
COVID-19 is primarily spread through inhalation of respiratory droplets and the most severely ill patients require a ventilator to help them breathe. Unlike invasive ventilators, which require intubation, non-invasive ventilators help patients breathe through a mask that provides positive pressure to keep the lungs open and functioning. While non-invasive ventilators may be effective for some COVID-19 patients, their use comes with a much higher risk of spreading infection through aerosolization of respiratory droplets.
The team’s non-invasive ventilation mask aims to eliminate this risk. The novel device is customized from a standard firefighter’s mask using 3D printing and can be attached to any CPAP or BiPAP machine. Unlike traditional masks, it creates two tight seals – one around the patient’s nose and mouth and another around the face. Patients breathe in and out of a filter that captures any viral particles before they are released to the air.
“There are countless CPAP and BiPAP machines idling around the world while all resources go towards invasive ventilation,” explains Dr. Azad Mashari, Anesthesiologist at UHN’s Peter Munk Cardiac Centre. “Our mask aims to put these machines back into the clinician’s toolkit. By eliminating air leaks, we can improve patient safety and significantly reduce the risk of contracting COVID-19 for health-care workers and other patients.”
Drs. Loubani, Mashari and Benjamin Thomson, Nephrologist at Mackenzie Health, were part of a clinical research team that worked with engineers from GDLS-Canada to develop the device within six days.
“GDLS-Canada responded quickly to the urgent need to support those on the COVID-19 healthcare frontlines during this global health emergency,” says Doug Wilson-Hodge, GDLS-Canada’s Manager of Communications, Community and Government Relations. “The innovative design was very much a collaborative effort between all parties to contribute solutions to the COVID-19 pandemic.”
The initial clinical trial will test the device with up to 50 patients at LHSC’s Victoria Hospital and University Hospital with plans to expand to UHN. In addition to patients with COVID-19, participants will include those with asthma, chronic obstructive pulmonary disease (COPD) and congestive heart failure (CHF).
The research team anticipates other hospitals in Ontario and across Canada will join the study to create a multi-centre clinical trial. The device will be used in emergency departments and has potential to be used in intensive care units, remote nursing stations and during pre-hospital transport. It has also been designed for easy production in resource-strained locations.
“This problem affects everyone and it’s critical that we all do what we can to help,” adds Dr. Loubani. “We hope it will help not only those in urban centres like Toronto and London, but people in remote communities around the world.”
The trial is being supported with funding from Glia, an organization internationally recognized for producing medical supplies that are easily accessible and can be manufactured in low-resource settings.
-30-
DOWNLOADABLE MEDIA
Images




Video



Lawson Health Research Institute is one of Canada’s top hospital-based research institutes, tackling the most pressing challenges in health care. As the research institute of London Health Sciences Centre and St. Joseph’s Health Care London, our innovation happens where care is delivered. Lawson research teams are at the leading-edge of science with the goal of improving health and the delivery of care for patients. Working in partnership with Western University, our researchers are encouraged to pursue their curiosity, collaborate often and share their discoveries widely. Research conducted through Lawson makes a difference in the lives of patients, families and communities around the world. To learn more, visit www.lawsonresearch.ca.
London Health Sciences Centre has been at the forefront of medicine in Canada for 145 years and offers the broadest range of specialized clinical services in Ontario. Building on the traditions of its founding hospitals to provide compassionate care in an academic teaching setting, London Health Sciences Centre is home to Children’s Hospital, University Hospital, Victoria Hospital, the Kidney Care Centre, two family medical centres, and two research institutes – Children’s Health Research Institute and Lawson Health Research Institute. As a leader in medical discovery and health research, London Health Sciences Centre has a history of over 70 international and national firsts and attracts top clinicians and researchers from around the world. As a regional referral centre, London Health Sciences Centre cares for the most medically complex patients including critically injured adults and children in southwestern Ontario and beyond. The hospital’s nearly 15,000 staff, physicians, students and volunteers provide care for more than one million patient visits a year. For more information, visit www.lhsc.on.ca.
University Health Network consists of Toronto General, recently voted one of the Top 5 Hospitals in the World according to Newsweek Magazine, and Toronto Western Hospital, the Princess Margaret Cancer Centre, Toronto Rehabilitation Institute, and the Michener Institute of Education at UHN. The scope of research and complexity of cases at University Health Network has made it a national and international source of discovery, education and patient care. It has the largest hospital-based research program in Canada, with major research in cardiology, transplantation, neurosciences, oncology, surgical innovation, infectious diseases, genomic medicine and rehabilitation medicine. University Health Network is a research hospital affiliated with the University of Toronto. www.uhn.ca
Senior Media Relations Consultant
Communications & Public Engagement
T: 519-685-8500 ext. 73502
Celine.zadorsky@lhsc.on.ca
New imaging solution could help improve survival for patients with recurring prostate cancer
London, ON - A multicentre study led by London Health Sciences Centre Research Institute (LHSCRI), Lawson Research Institute of St. Joseph’s Health Care London (Lawson), and University Health Network (UHN) has found a novel imaging solution, called prostate-specific membrane antigen (PSMA) positron emission tomography (PET) scanning, can more effectively detect the recurrence of prostate cancer compared to standard imaging methods, and is associated with improved survival outcomes. The study, carried out over seven years, is published in The Journal of Nuclear Medicine.
During a PSMA PET scan, a radioactive molecule designed to target a protein in prostate cancer cells is injected into the bloodstream of a patient prior to the scan. The study uncovered that the molecule is effective in binding to prostate cancer cells, helping to detect recurring prostate cancer earlier and more effectively than standard imaging methods.
“This new technique gives physicians the information needed to determine the best treatment,” says Dr. Glenn Bauman, Scientist at LHSCRI and Radiation Oncologist at London Health Sciences Centre (LHSC). “When a blood test shows cancer has returned but standard imaging can’t find it, physicians may need to use less precise therapies like whole-body drug therapy. With this new imaging technique, we can locate the cancer and target it directly.”
The research team found that the overall detection rate was 70 per cent, much higher than the historical rates of detection of 10-20 per cent with conventional bone scan and CT scans. About half of all patients had their management of the disease changed based on the results of the scans. Almost 90 per cent of men with cancer detected by PSMA PET had a change in management of their recurring prostate cancer following the scan. They also found that patients who had their treatments modified based on results from the PET scan had a better overall survival rate than those who received standard imaging.
“We’re encouraged by how this imaging approach is already changing cancer care,” says Dr. Ur Metser, Division Head of Molecular Imaging at UHN and Clinician Scientist at UHN’s Princess Margaret Cancer Centre. “Our study showed that PET scans using this technique led to more personalized treatment decisions and those changes are linked to longer survival. That’s a meaningful step forward for patients and their care teams.”
Dr. Bauman and his colleagues from Lawson and LHSCRI were the first in Canada to image a patient using PSMA PET imaging in 2016. Since then, this study has enrolled thousands of men across six hospitals in Ontario through funding from Ontario Health - Cancer Care Ontario. Based on promising results from this and other research, PSMA PET scans are now funded as a standard of care test for men with advanced prostate cancer.
For more information, please contact: Deb Flaherty, Consultant, Communications & Public Affairs, St. Joseph's Health Care London.
519-646-6100 ext. 47560
ABOUT LAWSON RESEARCH INSTITUTE
Lawson Research Institute, the health innovation arm of St. Joseph's Health Care London, is committed to making and sharing discoveries that improve lives locally and internationally. Every day, Lawson researchers work to transform imagination to innovation to patient impact. Lawson leads health-care research. Find us at LawsonResearch.ca and @stjosephslondon on social media.
ABOUT LONDON HEALTH SCIENCES CENTRE RESEARCH INSTITUTE
At London Health Sciences Centre Research Institute (LHSCRI), our teams pioneer discoveries that transform the health of adult and paediatric patients around the world. As the research institute of London Health Sciences Centre (LHSC), we conduct research where patient care is delivered, working alongside patients, families, health-care providers and academic partners like Western University. We are leaders in advancing the understanding, diagnosis, treatment and management of diseases and health conditions through a diverse research program that ranges from laboratory-based science to clinical trials. Our research has a global impact as we build on LHSC’s 150-year legacy of health innovation and drive forward medical breakthroughs that
make a difference in the lives of patients and their families. Find us online at WWW.LHSCRI.CA and on social media @LHSCRI.