Search
Search
History
Each hospital’s research mission has a rich history. At both hospital organizations, leaders recognized opportunities to leverage in-house experts to conduct research and improve care. However, they also recognized the challenge in supporting these activities without dedicated space and resources.
Through great foresight, our hospitals founded the official research institutes that serve as Lawson's foundation:
- 1983: Supported by Sister Mary Doyle, former Executive Director of St. Joseph's, the Sisters of St. Joseph's establish the hospital's official research institute. LHSC and Upjohn jointly open the Victoria Upjohn Clinical Research Unit at South Street Hospital (formerly Victoria Hospital), focusing on Phase I-III clinical trials.
- 1987: The St. Joseph's research institute is named the Lawson Research Institute (LRI) in honour of London businessman and philanthropist Colonel Tom Lawson and his wife, Miggsie Lawson - close friends of Sister Mary Doyle and major supporters of the research mission.
- 1990: Victoria Hospital takes over the operation of the clinical research unit at South Street, renaming it the Victoria Clinical Trials Centre.
- 1997: The Victoria Clinical Trials Centre is renamed London Health Sciences Centre Research Inc. and becomes a fully incorporated research institute overseeing all hospital-based research within London Health Sciences Centre sites: Victoria Hospital, University Hospital and South Street Hospital.
- 2000: LRI and LHSCRI merge to form a joint venture: Lawson Health Research Institute.
- 2014: Lawson Research Institute (re-)launches as the hospital-based research arm of St. Joseph's with the goal of transforming imagination to innovation to impact; and as LHSCRI is also embedded into LHSC.
Today, partnerships remain strong, allowing researchers to move seamlessly between hospital locations and Western University.
Milestones
Since forming in 2000, Lawson has pioneered breakthroughs across various disciplines of health research and reached several institutional milestones.
- 2019: Lawson led research team is the first in the world to develop a new imaging tool, showed that MRI can be used to measure how the heart uses oxygen.
- 2019: New studies from Lawson and Western University found for the first time that HIV can be transmitted through the sharing of equipment used to prepare drugs before injection and that a simple intervention can destroy the HIV virus, preventing that transmission.
- 2019: In the first genomic analysis of head and neck cancer by smoking status, researchers at Lawson, in collaboration with researchers at the Ontario Institute for Cancer Research and UCLA Cancer Centre, carried out a comprehensive genetic analysis of HPV-negative tumours to better understand the link between smoking and cancer recovery.
- 2019: Lawson scientists develop molecular diagnostic tool to analyze epigenetic patterns, facilitating diagnosis of rare, unknown hereditary disorders. London Health Sciences Centre is the first site in the world to offer this type of testing.
- 2018: Research shows high-dose radiation can improve survival in patients with cancer that has spread to give or less sites. The SABR-COMET study was the first randomized phase II clinical trial of its kind.
- 2018: An international collaborative study between Lawson Health Research Institute, Memorial Sloan Kettering Cancer Center, the Royal Marsden and Epic Sciences is one of the first to demonstrate that a blood test can predict how patients with advanced prostate cancer will respond to specific treatments, leading to improved survival.
- 2018: In collaborative study between Lawson and Stanford University, scientists develop and test a new synthetic surfactant that could lead to improved treatments for lung disease and injury.
- 2018: Scientists use brain MRI to develop first ever method examining young people before they become ill to reliably identify who will develop acute psychosis and who will not.
- 2018: Research team develops clinically-validated, open-source 3D printed stethoscope for areas with limited access to medical supplies.
- 2018: Lawson opens Clinical Research and Chronic Disease Centre (CRCDC) at St. Joseph’s Hospital to tackle chronic disease and improve patient care.
- 2018: Lawson researchers receive $4.4 million to study personalized medicine at LHSC, examining the value of prescribing treatments based on a patient’s genetics.
- 2017: In one of the largest microbiota studies conducted in humans, researchers at Western University, Lawson Health Research Institute and Tianyi Health Science Institute in Zhenjiang, Jiangsu, China have shown a potential link between healthy aging and a healthy gut.
- 2017: Lawson researchers develop transition program to help young adults with type 1 diabetes move from paediatric to adult care.
- 2017: Innovative study brings next-generation genome sequencing to London cancer patients, contributing to province-wide database of genomic and clinical data.
- 2017: Technology developed at Western University and Lawson Health Research Institute can provide a new window into whether or not patients are responding to treatment for advanced ovarian cancer.
- 2017: Dr. Alan Getgood and his team at Western University and Lawson Health Research Institute are the first in Canada to participate in an investigative trial to determine the safety and efficacy of using a patient’s own cartilage cells to repair knee cartilage injuries.
- 2016: Lawson Researchers at Parkwood Institute are the first in Canada to develop clinical practice guidelines for managing neuropathic pain with patients who have experienced a spinal cord injury.
- 2016: Researchers at Lawson are the first in Canada to use a Prostate Specific Membrane Antigen (PSMA) probe in Positron Emissions Tomography (PET) scans to provide improved and highly specific images used for better diagnosis and management of prostate cancer.
- 2015: Lawson scientists, in collaboration with Ceresensa Inc., produce novel PET-transparent MRI head coil, a world first in imaging technology
- 2015: Lawson announces partnership with STEMCELL Technologies for commercialization of tools for Parkinson’s disease research
- 2015: Novare Pharmaceuticals and Lawson announce issuance of a U.S. patent for the composition-of-matter and use of RHAMM-binding peptides with a wide range of potential therapeutic uses. The patent also has claims for the diagnosis and prognosis of cancer, and for prescribing a course of treatment for the diagnosed cancer.
- 2014: Lawson announces licensing agreement with Yabao Pharmaceutical Group in China to develop and test a new life-saving drug to treat sepsis
- 2014: Lawson researchers are part of a Canadian team who have developed a way to produce a key medical isotope, technetium-99m (Tc-99m), using hospital based cyclotrons
- 2013: The Institute for Clinical Evaluative Sciences (ICES) Western opens at Lawson
- 2012: Lawson installs Canada's first PET/MRI at St. Joseph's Hospital
- 2011: Lindros Legacy Research Building officially opens at University Hospital
- 2010: Lawson opens the Cyclotron and PET Radiochemistry facility at St. Joseph's Hospital
- 2009: Lawson receives a record $7 million donation to support the Canadian Research & Development Centre for Probiotics
- 2008: Lawson establishes an experimental anti-thrombolitic clinic to calculate personalized dosage of drugs based on a patient's genetics
- 2007: The first totally endoscopic closed-chest robotic coronary artery bypass surgery on a patient's beating heart is performed at University Hospital
- 2006: Lawson opens the Aging, Rehabilitation & Geriatric Care Research Centre, the first centre of its kind in Canada, at Parkwood Institute
- 2005: Lawson creates the first Ontario Cardiac Rehabilitation Registry
- 2004: Lawson scientists release a three-year study on the effects of the Walkerton water disaster
- 2003: Lawson opens the Victoria Research Laboratories at Victoria Hospital, the first collaboration of its kind in Canada bringing together research from cancer, children's health and vascular biology
- 2002: Lawson installs the first Positron Emission Tomography and Computer Tomography (PET/CT) scanner in Canada at St. Joseph's Hospital
- 2001: St. Joseph's is one of five sites in the world piloting the Diabetes Electronic Management Systems
Improving surgery for wrist arthritis
Wrist arthritis can cause debilitating pain, weakness and decreased range of motion. When patients are first diagnosed, the condition can often be managed with activity modification and pain medication. However, as symptoms progress, patients eventually require surgery.
Surgeons typically perform a procedure called four-corner fusion to preserve wrist motion and provide pain relief. This surgery involves removing one of the carpal bones and fusing four of the remaining carpal bones. Although this procedure is one of the most common treatments for wrist arthritis, it is not known how the position of the fusion of the wrist bones affects range of motion and joint contact.
Lawson associate scientist Dr. Nina Suh is leading a study with the goal of improving the surgical technique for four-corner fusion to maximize wrist function and symptom relief, and delay wrist arthritis progression.
Dr. Suh and her team will use a customized active-motion wrist simulator to create different carpal bone fusion positions. They will then assess how these positions affect wrist motion and joint contact area.
“We hope this research will lead to new surgical techniques that will help us to more effectively manage wrist arthritis with four-corner fusion,” says Dr. Suh, who is also an orthopaedic surgeon at the Roth McFarlane Hand and Upper Limb Centre (HULC) at St. Joseph’s Health Care London and an assistant professor at Western University’s Schulich School of Medicine & Dentistry. “The project will also advance our understanding of wrist biomechanics, providing a foundation for the development of enhanced patient-specific surgical tools, such as custom wrist fusion devices and implants.”
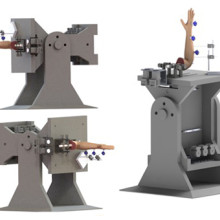
Image of the customized active-motion wrist simulator Dr. Nina Suh and her team are using to create different carpal bone fusion positions. They will then assess how these positions affect wrist motion and joint contact area.
The study is being funded through the Lawson Internal Research Fund (IRF), designed to allow scientists the opportunity to obtain start-up funds for new projects with exciting potential.
“The IRF program is valuable for scientists as external funding sources routinely require preliminary data to strengthen applications,” says Dr. Suh. “Particularly for new scientists such as myself, these grants provide seed funding that allows us to demonstrate the validity of our methodology and the clinical usefulness of our results.”
The IRF is designed to provide Lawson scientists the opportunity to obtain start-up funds for new projects with the potential to obtain larger funding, be published in a high-impact journal, or provide a clinical benefit to patients. Funding is provided by the clinical departments of London Health Sciences Centre and St. Joseph’s Health Care London, as well as the hospital foundations (London Health Sciences Foundation and St. Joseph’s Health Care Foundation).
Leveraging virtual reality to manage pain in paediatric patients
London - A new study underway through Lawson Health Research Institute and Children’s Hospital at London Health Sciences Centre (LHSC), using virtual reality (VR) to help pediatric patients during painful and distressing procedures.
“Technology holds immense potential for improving the experience of our young patients and their families,” explains Dr. Naveen Poonai, Lawson Scientist, principal investigator and Emergency Department Physician at Children’s Hospital. “VR is becoming increasingly popular amongst young people and some early research shows VR has been helpful in painful procedures, even in adults.”
The study is focusing on pediatric patients who need port access. A port is a little reservoir that sits underneath the skin that allows access to blood or medication with the use of a needle. Ports are most commonly used in pediatric cancer patients.
“This can be very distressing for a patient and it can set the tone for their entire clinic day and course of treatment,” says Dr. Alexandra Zorzi, Lawson Associate Scientist and Pediatric Oncologist at Children’s Hospital. “Minimizing the stress, anxiety, and pain of the procedure is key to avoiding a negative experience.”
The study team is recruiting 90 pediatric patients with existing medical ports. Participants will be randomized into three groups. One group will be using a VR headset that will allow them to play interactive games. The second group will have access to tablet technology, and the final group will be provided with non-technology distractions. Each procedure and the patient’s response will be recorded. Responses will then be compared using a tool called the ‘Observational Scale of Behavioral Distress’ to determine which intervention leads to the best outcomes.
“My hopes are that we develop a variety of skills we can tailor to patients,” adds Dr. Zorzi. “There are patients who receive all kinds of support but still struggle, so having a variety of techniques available to see what works best is a positive step forward.”
The study is expected to be completed by the end of this year. The research team is already collaborating with Children’s Hospital staff and leadership to use VR as a clinical tool if the study proves the technology to be effective.
“We have plans in place to allow whatever we find as the best option to be part of routine care for kids needing port access,” notes Dr. Poonai. “We are also speaking with various medical teams to determine how we can incorporate this into practices across the hospital.”
The use of the VR devices has been made possible with generous support from the Children’s Health Foundation.
Lawson Health Research Institute is one of Canada’s top hospital-based research institutes, tackling the most pressing challenges in health care. As the research institute of London Health Sciences Centre and St. Joseph’s Health Care London, our innovation happens where care is delivered. Lawson research teams are at the leading-edge of science with the goal of improving health and the delivery of care for patients. Working in partnership with Western University, our researchers are encouraged to pursue their curiosity, collaborate often and share their discoveries widely. Research conducted through Lawson makes a difference in the lives of patients, families and communities around the world. To learn more, visit www.lawsonresearch.ca.
Senior Media Relations Consultant
Communications & Public Engagement
T: 519-685-8500 ext. 73502
Celine.zadorsky@lhsc.on.ca
New Alzheimer’s research aims to improve treatment and support for patients with agitation
Two new interventional studies have been brought to London, focused on improving quality of life for patients with Alzheimer’s disease and their caregivers. Both hope to improve upon standard approaches to treating agitation, a core symptom of Alzheimer’s.
Agitation is a significant source of stress for patients and caregivers. It is complex and difficult to treat. Often, families do not know about this particular symptom of Alzheimer’s and are not properly trained on how to manage care while dealing with agitation.
“These studies are designed to have a direct impact on patients, families and care providers, to improve quality of life and function in those suffering from agitation due to Alzheimer’s,” says Dr. Amer Burhan, Associate Scientist at Lawson Health Research Institute (Lawson) and Geriatric Neuropsychiatrist, St. Joseph’s Health Care London (St. Joseph’s).
Parkwood Institute, a part of St. Joseph’s, is one of multiple sites participating in these studies across Canada and the United States.
One study aims to identify patients early in their diagnosis, while they are living at home or in the community, and apply a comprehensive psychosocial approach, with or without medication, to help with the management of agitation. “We hope to identify participants and have them participating in our program before they experience a crisis due to agitation,” explains Dr. Burhan.
Psychosocial intervention is a way of helping patients and caregivers understand the reasons for agitation. Agitation can develop due to a wide range of causes. For example, patients may just be bored and need help to find something meaningful to occupy their time, they could be upset about something in their current environment, or may be suffering from physical discomfort or pain.
Interventions can include communicating with patients in a manner that creates calm, scheduling meaningful activities, and maintaining routine and rhythm in life. The research team will connect with families early after diagnosis to give them the tools and support they need.
Initially, participants will be treated using structured psychosocial intervention to help reduce and manage their agitation. After three weeks, they will be reassessed and if significant agitation continues to persist, the patient will be randomly selected to receive either a placebo, or medication known as S-Citalopram to treat agitation while they continue to receive psychosocial care.
Sylvia Wilson is the wife of one of the study participants. By enrolling in this trial, she feels she has gained a much better understanding of her husband’s disease, and is grateful for the support that study participants receive.
“My husband typically does not like going to visit doctors, but Dr. Burhan and his team are great,” says Wilson. “They understand agitation, and other symptoms of the disease very well, and I notice a difference in his mood with the treatment he receives through the study.”
Participants are still able to receive care from their primary physician and care teams, with the study providing an added layer of support.
Another study is focused on Alzheimer’s patients who are admitted to hospital or living in long-term care. The aim is to standardize the approach to care for agitation related to Alzheimer’s. After baseline assessment, participants will be randomized to receive the current treatment as per usual, or an integrated care pathway derived from evidence-informed treatment guidelines. These include washing out medications that have not helped, adding individualized behavioral and environmental support, and if medications are needed, use a specific set of medications and dosages based on best evidence.
“Better understanding agitation is a growing area of interest in geriatric research. The work being done locally is part of an international effort to create a paradigm shift in treating patients with Alzheimer’s disease and agitation,” explains Dr. Burhan.
Researchers are ready to offer these studies to patients and their families, hoping to make these treatment protocols an integral part of care for patients with agitation due to Alzheimer’s disease. Those interested in learning more about these studies can contact Dr. Burhan at @email or call 519-646-6100 x. 48170.
In the media: Study on 'agitation' in Alzheimer's patients seeks participants