Search
Search
Celebrating Clinical Trials Day
Clinical trials are the gold standard in medical research, used to test new treatments and medical devices to ensure they are safe and improve patient outcomes.
Each year on May 20, Clinical Trials Day aims to raise awareness about the importance of clinical trials. At Lawson Health Research Institute, our researchers, research staff and learners across London Health Sciences Centre (LHSC) and St. Joseph’s Health Care London (St. Joseph’s) are working daily to advance clinical trials for some of the most pressing health challenges.
“If you look at many areas of medicine, like cancer and cardiovascular disease, part of why those conditions have had dramatic improvements in outcomes over the last several decades is because of clinical trials,” says Dr. Amit Garg, Scientist at Lawson, Lead for the Kidney, Dialysis & Transplantation Research Program at ICES Western, and a Nephrologist at LHSC.
Clinical trials can also provide patient participants with new treatment options and can demonstrate when existing treatments have applications for other diseases.
“We could not conduct clinical trials without patients participating in them,” adds Dr. David Palma, Associate Scientist at Lawson and Radiation Oncologist at LHSC. “A clinical trial is a very rigorous process where we carefully define a treatment and follow patients very closely with extra interventions and tests to see not only how the disease is responding to treatment, but also any effects on a patient’s quality of life.”
It also takes a team to make clinical trials a success, including the critical work of research coordinators, associates and assistants, adds Dr. Swati Mehta, Lawson Scientist based at St. Joseph’s Parkwood Institute.
Dr. Palma also notes that while clinical trials require investment to conduct them, they can ultimately lead to savings in the health system.
“While the primary goal of a clinical trial is to improve or save lives, they often lead to cost savings down the road. Improving cure rates means people don’t need as much treatment and that can save the initial investment many, many times over,” Palma says.
Looking ahead, work is ongoing to make clinical trials more efficient and equitable.
“Eliminating specialized infrastructure would help make trials more equitable, so they are available in smaller communities and at distant sites that otherwise would not have access. Making study materials available in multiple languages and to anyone with accessibility issues can also help,” Garg adds.
“Future clinical trials will need to follow more pragmatic, adaptive study designs that allow us to evaluate therapies or interventions in a more realistic setting,” Dr. Mehta says. “These would also allow us to follow-up with patients that were potentially underrepresented in past research.”
According to researchers at Lawson, the future of clinical trials is bright with hundreds of trials currently underway at LHSC and St. Joseph’s with the goal of improving patient outcomes.
Celebrating remarkable women in science
Chronic pain can affect every facet of a person’s life. “When someone is in pain, they can have significant difficulty with activities we all do in our daily life, from getting out of bed to walking to the mailbox. It can also impact their mental wellness,” shares Lawson Health Research Institute Scientist Dr. Siobhan Schabrun, PhD. Dr. Schabrun, who is the first ever William and Lynne Gray Endowed Research Chair in Mobility and Activity at St. Joseph’s Health Care London, has dedicated much of her career to unravelling the complex connection between the body and the mind known as neuroscience, with a focus on persistent pain.
She is currently leading groundbreaking work in understanding, treating and preventing persistent pain, offering hope for enhanced mobility and activity in individuals with musculoskeletal and neurological conditions at St. Joseph’s Gray Centre for Mobility and Activity, located at Parkwood Institute.
Dr. Schabrun’s research program has extended beyond conventional approaches, delving into the use of non-invasive brain stimulation technologies such as repetitive transcranial magnetic stimulation, more commonly known as rTMS, a therapeutic tool used in mental health treatments for decades, to augment neuroplasticity and optimize outcomes in rehabilitation.
Bridging the gap between human pain models and clinical trials, Dr. Schabrun’s work is contributing to the understanding of clinical pain populations and bringing new treatment methods to the forefront to improve patient care and outcomes.

Clinical trial will evaluate new therapy for patients with treatment-resistant depression as a result of bipolar disorder
LONDON, ON – Researchers at Lawson Health Research Institute are offering new hope to patients with treatment-resistant depression through participation in a national clinical trial. The study is the first randomized controlled trial to examine the efficacy of a new treatment called magnetic seizure therapy (MST) for patients with treatment-resistant depression as a result of bipolar disorder.
Treatment-resistant depression is a severe form of depression that does not respond to traditional therapies like medication. It is particularly common in patients with depression from bipolar disorder who are left with limited treatment options.
For years electroconvulsive therapy (ECT) has been the gold standard for patients with treatment-resistant depression. ECT uses an electric field to induce a seizure that provides a therapeutic benefit. But while ECT is effective, many patients opt out of treatment due to stigma surrounding the therapy and the potential for cognitive side effects like disorientation and amnesia.
MST has emerged as a promising alternative. MST works in a similar way to ECT but uses a focused magnetic field as opposed to electricity. As a result, MST induces a more focused seizure to reduce the risk of cognitive side effects.
“Magnetic seizure therapy has already been shown as a promising treatment for major depressive disorder or unipolar depression,” explains Dr. Amer Burhan, local site lead, researcher at Lawson and neuropsychiatrist at St. Joseph’s Health Care London. “For the first time, we’re studying how effective the treatment is for depression as a result of bipolar disorder and whether it can reduce the risk of cognitive side effects associated with electroconvulsive therapy.”
The clinical trial is being led by the Centre for Addiction and Mental Health (CAMH) and will also be offered through Lawson and University of British Columbia (UBC) Hospital. Lawson researchers will invite eligible patients with treatment-resistant depression from bipolar disorder to participate in the trial at Parkwood Institute, a part of St. Joseph’s Health Care London.
Eligible patients will be randomized to receive either ECT or MST. Patients will be offered support throughout the study with the goal of improving patient outcomes in both groups. Patient outcomes will be compared to study the efficacy of MST and whether it is associated with reduced cognitive side effects.
MST will be delivered under anesthesia in 12 to 20 sessions. Sessions will last 10 to 15 minutes each with 60 to 90 minutes of recovery time.
“Magnetic seizure therapy holds promise of one day replacing electroconvulsive therapy as the gold standard for treatment-resistant depression,” says Dr. Burhan. If proven as a viable first-line treatment, MST would be very easy to implement in existing ECT clinics. MST would therefore be readily available to patients in need.
“We are on the leading edge of the field of brain stimulation for treatment-resistant depression in collaboration with CAMH and UBC,” says Dr. Burhan. “Our goal is to continue informing the care process through clinically-relevant research that serves patients, medical professionals and the public.”
-30-
Lawson Health Research Institute is one of Canada’s top hospital-based research institutes, tackling the most pressing challenges in health care. As the research institute of London Health Sciences Centre and St. Joseph’s Health Care London, our innovation happens where care is delivered. Lawson research teams are at the leading-edge of science with the goal of improving health and the delivery of care for patients. Working in partnership with Western University, our researchers are encouraged to pursue their curiosity, collaborate often and share their discoveries widely. Research conducted through Lawson makes a difference in the lives of patients, families and communities around the world. To learn more, visit www.lawsonresearch.ca.
Senior Media Relations Consultant
Communications & Public Engagement
T: 519-685-8500 ext. 73502
Celine.zadorsky@lhsc.on.ca
Clinical trial will evaluate new therapy for treatment-resistant depression as a result of bipolar disorder
Treatment-resistant depression is a severe form of illness that does not respond to traditional therapies like medication and counselling. It is particularly common in patients with depression from bipolar disorder who are left with limited treatment options.
“There are some mental illnesses that can become resistant to therapy, similar to how infections, for example, can become resistant to antibiotics,” explains Dr. Amer Burhan, researcher at Lawson Health Research Institute and neuropsychiatrist at St. Joseph’s Health Care London. “Patients with those illnesses need more options.”
Brain stimulation is a field that holds promise for this patient population.
“When people are in a state of depression, research shows their brain networks are not functioning properly,” says Dr. Burhan. “Brain stimulation aims to stimulate neurons in the brain to correct activity and improve patient outcomes.”
Through involvement in a national clinical trial, Dr. Burhan and his research team at Lawson are offering new hope with a treatment called magnetic seizure therapy (MST). The study is the first randomized controlled trial to examine the efficacy of MST for patients with treatment-resistant depression as a result of bipolar disorder.
For years electroconvulsive therapy (ECT), one form of brain stimulation, has been the gold standard for patients with treatment-resistant depression. ECT uses an electric field to induce a seizure that provides a therapeutic benefit by stimulating the brain. But while ECT is effective, many patients opt out of treatment due to stigma surrounding the therapy and its potential for cognitive side effects like disorientation and amnesia.
MST has emerged as a promising alternative. MST works in a similar way to ECT but uses a focused magnetic field as opposed to electricity. As a result, MST induces a more focused seizure to reduce the risk of cognitive side effects.
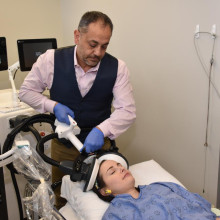
“Magnetic seizure therapy has already been shown as a promising treatment for major depressive disorder or unipolar depression,” explains Dr. Amer Burhan, local site lead for the clinical trial. “For the first time, we’re studying how effective the treatment is for depression as a result of bipolar disorder and whether it can reduce the risk of cognitive side effects associated with electroconvulsive therapy.”
The clinical trial is being led by the Centre for Addiction and Mental Health (CAMH) and will also be offered through Lawson and University of British Columbia (UBC) Hospital. Lawson researchers will invite approximately 30 eligible patients with treatment-resistant depression from bipolar disorder to participate in the trial at Parkwood Institute, a part of St. Joseph’s Health Care London.
Eligible patients will be randomized to receive either ECT or MST. Patients will be offered support throughout the study with the goal of improving patient outcomes in both groups. Patient outcomes will be compared to study the whether MST is effective and whether it is associated with reduced cognitive side effects.
MST will be delivered under anesthesia in 12 to 20 sessions. Sessions will last 10 to 15 minutes each with 60 to 90 minutes of recovery time.
“Magnetic seizure therapy holds promise of one day replacing electroconvulsive therapy as the gold standard for treatment-resistant depression, but we need to learn more about where it fits in our toolbox of potential treatments,” says Dr. Burhan.
If proven as a viable first-line treatment, MST would be very easy to implement in existing ECT clinics. MST would therefore be readily available to patients in need.
“We are on the leading edge of the field of brain stimulation for treatment-resistant depression in collaboration with CAMH and UBC,” says Dr. Burhan. “Our goal is to continue informing the care process through clinically-relevant research that serves patients, medical professionals and the public.”
Those who would like more information about the trial can email @email or @email.