Search
Search
Research Bites: Why it is important to keep moving as we age
Dr. Tim Doherty is a clinician scientist at Lawson’s Parkwood Institute Research with a primary focus on the impact of aging on nerve and muscle function. He will discuss the impact of aging on mobility, why this is important, and how we can improve or prevent loss of mobility in older adults.
Virtual Event Details
Speaker: Dr. Tim Doherty
Date: Monday, November 9, 2020
Time: 4 - 5 p.m.
Location: This will be a virtual event hosted as a WebEx video meeting.
Meeting number (access code): 172 547 4015
Meeting password: M7JJdJA3
If joining by phone: call 519-685-8100
*No registration is required for this free public event.
About Research Bites
Presented by Parkwood Institute Research, a program of Lawson Health Research Institute, these informative and interactive talks focus on specific illnesses, their prevention and related research being conducted by researchers in London and area.
Research Data Management
Research data management (RDM) is the organization and maintenance of research data throughout the entire research project life cycle. This includes setting up protocols before initiating data collection, and then collecting, tracking, and creating backups of the data during study execution. It also includes data sharing, archiving and publishing upon project completion.
RDM is not a new concept. Lawson Research Institute (Lawson) of St. Joseph’s Health Care London researchers already employ these processes and procedures, and perform RDM in varying capacities.
Why is it important?
RDM is an essential part of research excellence. Research must be conducted to the highest professional standard by ensuring that it is performed ethically, makes good use of public funds, experiments and studies are replicable, and research results are as accessible as possible. In addition, some journals require certain types of data to be shared or stored in specific repositories as a condition of publication. Concerns around reproducibility of research results have led to increased interest in data sharing so research results can be replicated and confirmed.
There is also a need to elevate the availability of Canadian data on the world stage. This means we need more Canadian datasets to be cited and used in research outputs and acknowledged appropriately. This would also increase the ability for research data to be archived, found, and responsibly reused, to fuel new discoveries and innovation across multiple disciplines and geographical borders.
In short, strong RDM practice is a sign of research excellence and Lawson is committed to the highest quality of research integrity and excellence.
Institutional RDM Strategy
An institutional RDM strategy is a concise and directive document that outlines how an institution will increase its capacity for effective RDM.
The purpose of creating and establishing an institutional RDM strategy is to foster a culture of sustainable and collaborative data stewardship and develop the capacity to support researchers in adopting responsible RDM practices, following FAIR (Findable, Accessible, Interoperable, and Reusable) guiding principles.
Lawson recognizes that efficient research data management is an essential element of research excellence. A Lawson Institutional Research Data Management Strategy has been developed in accordance with the Tri-Agency Research Data Management Policy (Government of Canada, 2021).
For additional information please contact: @email.
Research Students: Required e-Learning
Lawson Research and Work Study Students can find their education modules below.
Please note that you may not be required to complete all the training on this page.
Please refer to the email you received from Research Health and Safety for detailed instructions on what training to compete.
All health and safety training requirements must be completed before your research placements/positions begin.
A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | U | V | W | X | Y | Z | IP students | Site specific
A
B
C
D
E
F
- Fire Safety and Extinguishers
- Infection Control Core Competency: Additional Precautions
- Infection Control Core Competency: Hand Hygiene
- Infection Control Core Competency: Routine Practices
- Influenza Prevention: Understanding Influenza and Influenza Vaccination
- Infomed (NOT required for students placed at Mount Hope)
- Infusion Pump Safety - Baxter module
- Intravenous Infusion
- IP Nursing - CADD Solis Infusion Pump
L
M
- Medical Device Reprocessing Competency Program (search in LearningEdge)
- Musculoskeletal Injury Prevention
O
P
- Preventing Falls and Injuries - Clinical (Inpatient areas)
- Preventing Falls and Injuries - Ambulatory (Outpatient areas)
- Privacy and Confidentiality
R
S
- Safe Delivery and Administration of 0-15 Gas
- Safety for Isotope Handlers
- Sexual Health Practice in Rehabilitation - Introduction
- Sexual Health Practice in Rehabilitation - Application to Clinical Practice
- Sharps Safety
- Slips, Trips and Falls
- Sterile Processing Competency Self-Assessment
- Suicide Risk Assessment and Prevention
T
U
V
W
- Working Safely with Chemicals
- Workplace Hazardous Materials Information System (WHMIS)
- Workplace Violence Prevention
X
Y
Z
Integrated Practicum (IP) students
- Accu-Chek Inform II Glucose Meter training
- Pyxis Competency Checklist
- Level 1 Vascular Access and Infusion Management: Assessment, Care and Maintenance
Site Specific
- Honeywell Personal Staff Alert Device Operation (any mental health care site)
- Prevention and Intervention in Crisis Situations (any mental health care site)
- Eliminating Abuse and Neglect in Long Term Care (Mount Hope only)
- Suicide Risk Assessment (program dependent - check the Required Learning Chart by Student Role)
Please refer to the email you received from Lawson Health and Safety for instructions on what training to complete.
You are required to complete your training before your research placements/positions begin.
Lawson Research Required Learning
SECTION A – Hospital Mandated Training:
- Behaviour Safety Alert
- Civility in the Workplace
- Cybersecurity
- Donning and Doffing of Surgical Masks (video)
- Emergency Colour Codes
- Emergency Eye Wash and Safety Showers
- Fire Safety and Extinguishers
- Honeywell Personal Staff Alert Device Operation (if you are given a device)
- Infection Control Core Competency: Hand Hygiene
- Infection Control Core Competency: Routine Practices
- Infection Control Core Competency: Additional Precautions
- Influenza Prevention
- Musculoskeletal Injury Prevention
- Privacy and Confidentiality
- Sharps Safety
- Slips, Trips and Falls
- Workplace Violence Prevention
Western certificates (OWL) accepted for the training below:
- AODA: Breaking Barriers: Your Guide to Understanding Accessibility
- Occupational Health and Safety Awareness Training
- Workplace Hazardous Materials Information System (WHMIS)
SECTION B – Basic Research Training & Documents/Policies
- Preventing Falls and Injuries - Non-clinical
- The Canadian Biosafety Standard (CBS) Second Edition
- Working Safely with Chemicals
Western certificates (OWL) accepted for the training below:
SECTION C – Clinical Research Specific Training & Documents/Policies
- Standard Operating Procedures for Clinical Research
- TCPS2 (Tri-Council Policy Statement 2)
Create your own account and login. Your affiliation should be with Lawson Health Research Institute.
Additional Documents, Policies and Training
Review if you are 25 years of age or under:
- Ontario Ministry of Labour information and tip sheets:
- Young Workers on the Job information or you can also download
RADIATION: OXYGEN-15 GAS TRAINING
Do not complete this training unless assigned by Lawson Health and Safety
Researchers developing photoacoustic hand-held probe for tumour detection during breast conserving surgery
Researchers at Lawson Health Research Institute (Lawson) are developing a hand-held photoacoustic imaging probe to be used during breast conserving surgery to quickly and accurately verify if all cancerous tissue has been removed.
Surgeons currently do not have real-time technology to guide tumour removal during surgery.
Using current tools, there is a 20 per cent chance that cancerous cells will be left behind, risking recurrence and repeat surgery.
Breast cancer represents 25 per cent of all new cancer diagnoses in women and 13 per cent of all cancer related deaths in women. Treatment for breast cancer often requires either complete breast removal in severe cases, or surgical removal of the cancerous tumour in combination with other therapies. Removing only the tumour is called breast conserving surgery.
Image


Photoacoustic Screening
The new device is an extension of the photoacoustic screening (iPAS) technology developed in the laboratory of Dr. Jeffrey Carson, Principal Investigator and Lawson Scientist. The technique uses light and sound to capture 3D images of surgically removed breast tissue. Their studies show that iPAS can catch up to 75 per cent of missed tumour cells, decreasing the odds of failed surgery to five per cent.
Dr. Muriel Brackstone, Associate Scientist at Lawson, Head of the Breast Care Clinic at St. Joseph’s Hospital London, and Surgical Oncologist at London Health Sciences Centre, brings her clinical expertise to the project.
“With the first generation iPAS technology, we would remove the tumour, take it to the lab for imaging and wait to see if there was a rim of normal tissue around the removed tumour so we knew it was removed completely. The wait was anywhere from 20 minutes to an hour. During that time, the patient is under anesthesia, the surgical team is idle and precious OR time is being used,” explains Dr. Brackstone.
A hand-held tool that surgeons can use
Creation of a hand-held probe to be used in the operating room is the next step in the advancement of this new technology. Elina Rascevska, biomedical engineering student at Western University, recently joined the Lawson team to convert lab-based iPAS technology into a hand-held device.
“We have developed a prototype of the iPAS probe, and once we can verify the quality of the images it produces, we will give it to Dr. Brackstone to test in the OR,” says Rascevska.
The iPAS probe does not need a trained operator and would be used by the surgical team. Instead of imaging the removed tissue, it scans the surgical cavity in real time to give the team a faster and more accurate indication as to whether the cancerous tissue has been removed.
“If we can progress this technology to a point where physicians can use it as part of standard protocols, we will have reduced the amount of time each patient needs to spend in the OR, the amount of call-backs and repeat surgeries, and ultimately improve quality of life for patients with breast cancer,” adds Dr. Carson.

(From left): Dr. Jeffrey Carson, Elina Rascevska, Dr. Muriel Brackstone
Researchers investigate a new method of sedation for paediatric patients
Scientists at Children’s Health Research Institute (a program of Lawson Health Research Institute), Sunnybrook Research Institute and The Hospital for Sick Children (SickKids) are working together to study the potential benefits of inhaled sedation as an alternative to keep critically ill children sedated and comfortable.
“Many sick children need support from a ventilator and other life-saving treatments, and may require intravenous (IV) sedatives to tolerate these uncomfortable therapies,” says Dr. Rishi Ganesan, Lawson Associate Scientist and Paediatric Neurocritical Care Physician at Children’s Hospital at London Health Sciences Centre (LHSC). “However, our current sedation options may contribute to a complication called delirium. We are interested in evaluating if delirium and long-term neurological complications are lower in children receiving inhaled sedation compared to those receiving IV sedation, which is the current standard of care.”
Delirium is an acute change in mental state that children in critical care can sometimes develop as a result of their critical illness and the medications and therapies they receive during their hospital stay. Delirium presents as confusion, disorientation, agitation, excessive drowsiness or poor attention. Dr. Marat Slessarev, Lawson Scientist and Critical Care Physician at LHSC, has been researching and comparing inhaled sedation to IV sedation in adults since the pandemic hit in 2020 in a collaborative trial called SAVE-ICU with Dr. Angela Jerath, Anesthesiologist and Scientist at Sunnybrook.
“One of the challenges with IV sedation is that we do not have a way to measure the level of sedatives in the blood,” explains Dr. Slessarev. “Critically ill patients that are sedated can sometimes develop issues with the kidney and liver, which are both important in eliminating the sedatives from the blood stream.”
Through this novel collaborative research, the team is now looking at the potential benefits of inhaled sedation in paediatric patients.
“Inhaled sedatives are an alternative to currently used IV sedatives, and they may reduce delirium and accelerate brain recovery. Inhaled sedatives are used safely every day in operating rooms, widely available and inexpensive,” explains Dr. Jerath. “In contrast to IV sedatives, they do not accumulate in the body, are rapidly eliminated via the lungs, promote faster awakening and discharge from a ventilator, and reduce inflammation – which may be a contributing factor to delirium.”
Enrollment for the ABOVE trial is beginning at Children’s Hospital at LHSC and SickKids. The pilot study will enroll 60 critically ill paediatric patients who will be randomized into two groups; one group will receive inhaled sedation while the other will get standard IV sedation. Once the pilot phase of the trial is complete, the team hopes to expand this trial across the country with more paediatric intensive care units (ICUs) joining the larger trial.
“The field of critical care has made significant strides in life-saving technologies and therapies in recent years, but now we are focused on finding ways to ensure our patients continue to do well after leaving the hospital,” says Dr. Nicole McKinnon, Critical Care Physician and lead investigator at SickKids and a Scientist Track Investigator at SickKids Research Insitute. “This trial is a first step in better understanding the effects of sedative and pain medications on children’s longer-term neurocognitive development. Our research will be key to providing critically ill children with the greatest chance to flourish at home.”
“This has the potential to change how critically ill children are cared for in paediatric ICUs across Canada and the world,” adds Dr. Ganesan. “We hope that inhaled sedation makes a difference in children’s long-term functional outcomes, so they can thrive and achieve their full potential.”
The ABOVE Trial recently received funding through a Canadian Institute for Health Research (CIHR) grant.
About Sunnybrook Research Institute: Sunnybrook Research Institute (SRI) is the research arm of Sunnybrook Health Sciences Centre, an internationally recognized academic health sciences centre fully affiliated with the University of Toronto. With well-established programs in basic and applied sciences which span across three scientific platforms and ten clinical programs, SRI is developing innovations in care for the more than 1.3 million patients the hospital cares for annually. To learn more, visit www.sunnybrook.ca/research
About The Hospital for Sick Children: The Hospital for Sick Children (SickKids) is recognized as one of the world’s foremost paediatric health-care institutions and is Canada’s leading centre dedicated to advancing children’s health through the integration of patient care, research and education. Founded in 1875 and affiliated with the University of Toronto, SickKids is one of Canada’s most research-intensive hospitals and has generated discoveries that have helped children globally. Its mission is to provide the best in complex and specialized family-centred care; pioneer scientific and clinical advancements; share expertise; foster an academic environment that nurtures health-care professionals; and champion an accessible, comprehensive and sustainable child health system. SickKids is a founding member of Kids Health Alliance, a network of partners working to create a high quality, consistent and coordinated approach to paediatric health care that is centred around children, youth and their families. SickKids is proud of its vision for Healthier Children. A Better World.
Lawson Health Research Institute is one of Canada’s top hospital-based research institutes, tackling the most pressing challenges in health care. As the research institute of London Health Sciences Centre and St. Joseph’s Health Care London, our innovation happens where care is delivered. Lawson research teams are at the leading-edge of science with the goal of improving health and the delivery of care for patients. Working in partnership with Western University, our researchers are encouraged to pursue their curiosity, collaborate often and share their discoveries widely. Research conducted through Lawson makes a difference in the lives of patients, families and communities around the world. To learn more, visit www.lawsonresearch.ca.
Communications Consultant & External Relations
Lawson Health Research Institute
T: 519-685-8500 ext. ext. 64059
C: 226-919-4748
@email
Researchers to study inhaled sedatives as solution to COVID-19 drug shortages
A team from Lawson Health Research Institute are being funded by the Government of Ontario’s COVID-19 Rapid Research Fund to study whether inhaled sedatives can replace those that are delivered intravenously in COVID-19 patients requiring ventilation. The multi-centre clinical trial aims to address a global shortage of intravenous (IV) sedatives while improving patient outcomes.
“When COVID-19 patients develop severe respiratory failure and need to be ventilated, they require sedation. While IV sedatives are currently used, there is concern about global drug shortages, particularly if there’s a second wave of COVID-19 in the fall,” explains Dr. Marat Slessarev, Scientist at Lawson and Critical Care Physician at London Health Sciences Centre (LHSC). “Even if we have enough ventilators, we won’t be able to ventilate patients without sedatives.”
The clinical trial, being co-led by Dr. Slessarev and Dr. Angela Jerath at Sunnybrook Health Sciences Centre, will study the replacement of IV sedatives with inhaled sedatives. Inhaled sedatives, also called volatiles, are widely available due to their use in operating rooms to sedate patients during surgery. While they have not been routinely used to sedate patients needing ventilation, early studies suggest they could be safe and even more effective than IV sedatives.
“Preliminary studies in non-COVID patients with severe respiratory failure suggest that inhaled sedatives can reduce lung inflammation, shorten the duration of ventilation and potentially improve survival. Inhaled sedatives could therefore reduce the pandemic’s strain on ventilator capacity while improving patient outcomes,” says Dr. Slessarev. “Since these drugs are safe, cheap and readily available, they can easily be used to address IV sedative shortages if found effective.”
The researchers will recruit approximately 800 patients from across Canada and the United States including patients from LHSC. Each patient will be randomized to receive either IV sedatives or inhaled sedatives. Patient outcomes such as survival and length of ventilation will be compared between the two groups to determine which method of sedation is most effective.
“This is the largest trial of its kind. If inhaled sedatives can shorten the length of ventilation or improve survival in patients with serve respiratory failure, this could cause a paradigm shift in the way we sedate patients in intensive care units (ICUs) around the world,” notes Dr. Slessarev.
Given many survivors of critical illness experience cognitive impairment for months or even years after an intensive care unit (ICU) stay, the team is also planning a sub-study to assess whether one method of sedation results in better cognitive outcomes after treatment.
In addition to funding from the Government of Ontario, the study is being supported with funding from the Canadian Institutes of Health Research (CIHR), London Health Sciences Foundation and Sunnybrook Health Sciences Centre.

Dr. Marat Slessarev, Scientist at Lawson and Critical Care Physician at LHSC
Researchers to study inhaled sedatives as solution to COVID-19 drug shortages
LONDON, ON - A team from Lawson Health Research Institute are being funded by the Government of Ontario’s COVID-19 Rapid Research Fund to study whether inhaled sedatives can replace those that are delivered intravenously in COVID-19 patients requiring ventilation. The multi-centre clinical trial aims to address a global shortage of intravenous (IV) sedatives while improving patient outcomes.
“When COVID-19 patients develop severe respiratory failure and need to be ventilated, they require sedation. While IV sedatives are currently used, there is concern about global drug shortages, particularly if there’s a second wave of COVID-19 in the fall,” explains Dr. Marat Slessarev, Scientist at Lawson and Critical Care Physician at London Health Sciences Centre (LHSC). “Even if we have enough ventilators, we won’t be able to ventilate patients without sedatives.”
The clinical trial, being co-led by Dr. Slessarev and Dr. Angela Jerath at Sunnybrook Health Sciences Centre, will study the replacement of IV sedatives with inhaled sedatives. Inhaled sedatives, also called volatiles, are widely available due to their use in operating rooms to sedate patients during surgery. While they have not been routinely used to sedate patients needing ventilation, early studies suggest they could be safe and even more effective than IV sedatives.
“Preliminary studies in non-COVID patients with severe respiratory failure suggest that inhaled sedatives can reduce lung inflammation, shorten the duration of ventilation and potentially improve survival. Inhaled sedatives could therefore reduce the pandemic’s strain on ventilator capacity while improving patient outcomes,” says Dr. Slessarev. “Since these drugs are safe, cheap and readily available, they can easily be used to address IV sedative shortages if found effective.”
The researchers will recruit approximately 800 patients from across Canada and the United States including patients from LHSC. Each patient will be randomized to receive either IV sedatives or inhaled sedatives. Patient outcomes such as survival and length of ventilation will be compared between the two groups to determine which method of sedation is most effective.
“This is the largest trial of its kind. If inhaled sedatives can shorten the length of ventilation or improve survival in patients with serve respiratory failure, this could cause a paradigm shift in the way we sedate patients in intensive care units (ICUs) around the world,” notes Dr. Slessarev.
Given many survivors of critical illness experience cognitive impairment for months or even years after an intensive care unit (ICU) stay, the team is also planning a sub-study to assess whether one method of sedation results in better cognitive outcomes after treatment.
In addition to funding from the Government of Ontario, the study is being supported with funding from the Canadian Institutes of Health Research (CIHR), London Health Sciences Foundation and Sunnybrook Health Sciences Centre.
-30-
DOWNLOADABLE MEDIA

Dr. Marat Slessarev, Scientist at Lawson Health Research Institute and Critical Care Physician at London Health Sciences Centre (LHSC)
Lawson Health Research Institute is one of Canada’s top hospital-based research institutes, tackling the most pressing challenges in health care. As the research institute of London Health Sciences Centre and St. Joseph’s Health Care London, our innovation happens where care is delivered. Lawson research teams are at the leading-edge of science with the goal of improving health and the delivery of care for patients. Working in partnership with Western University, our researchers are encouraged to pursue their curiosity, collaborate often and share their discoveries widely. Research conducted through Lawson makes a difference in the lives of patients, families and communities around the world. To learn more, visit www.lawsonresearch.ca.
Senior Media Relations Consultant
Communications & Public Engagement
T: 519-685-8500 ext. 73502
Celine.zadorsky@lhsc.on.ca
Researching treatments for COVID-19
As we continue to live with a COVID-19 pandemic, patients will need good treatment options.
Hospital researchers in London, Ontario, through Lawson Health Research Institute, are testing treatment options for patients who have been hospitalized from a COVID-19 infection, often for severe symptoms.
During the peaks of the COVID-19 pandemic, there were concerns of a global drug shortage when it came to IV sedatives for patients needing ventilation. Through funding from the Government of Ontario’s COVID-19 Rapid Research Fund, a team of Ontario researchers studied whether inhaled sedatives could replace those that are delivered through IV.
“In addition to easing the burden on IV stocks, the inhaled sedatives have additional benefits,” says Dr. Marat Slessarev, a Critical Care Physician at LHSC. “They may reduce inflammation in the lungs and shorten the duration of sedation because they are eliminated from the body faster than IV sedatives.”
Dr. Slessarev, also a Scientist at Lawson, adds that using inhaled sedatives could also be safer for severe COVID-19 patients, who in many cases are on a ventilator for a long time. “When using IV sedatives, they can accumulate and metabolize with accumulation. This can leave the patient with the potential of developing kidney issues because it is hard to dispel them quickly.”

Dr. Marat Slessarev, Critical Care Physican at LHSC and Lawson Scientist
To date, around 800 patients across ten hospital sites have been enrolled in this trial, with the hopes of getting more hospital sites on board as the clinical trials continue.
A majority of people with severe COVID-19 infections in critical care end up developing sepsis, which is a potentially life-threatening condition that occurs when the body's response to an infection damages its own tissues.
“An inflammatory response is what the body uses to fight an infection, but sometimes it is more than necessary and it causes damage to the organs and the body,” explains Dr. Claudio Martin, a Physician in the Intensive Care Unit (ICU) at London Health Sciences Centre (LHSC). “This is why a patient with a severe COVID-19 infection caused by a virus can get sepsis.”
Dr. Martin, who is also an Associate Scientist at Lawson, began studying the use of a human protein called Annexin A5 as a potential treatment for COVID-19 patients with sepsis. “It can work in two ways. The annexin may coat injured cells and reduce the inflammatory response. Or, the injured cells and exposed proteins trigger the clotting mechanism in the body. Those with COVID-19 could get blood clots in the brain and the lungs, and using annexin may improve their outcomes.”

Dr. Claudio Martin, Critical Care Physician at LHSC and Lawson Scientist
Currently, a clinical trial using Annexin A5 on severe COVID-19 patients is underway at LHSC to look at the efficacy of using this human protein.
The COVID-19 virus is also known to cause respiratory failure, prompting Dr. Jim Lewis, Respirologist at St. Joseph’s Health Care London and Scientist at Lawson, to investigate the use of pulmonary surfactant as a potential treatment for these patients.
Bovine Lipid Extract Surfactant Suspension (BLES) is a pulmonary surfactant manufactured in London, Ontario. It’s currently used worldwide to help improve lung function in premature babies. Now, it is being studied with COVID-19 patients experiencing respiratory failure.

Dr. Jim Lewis, Repirologist at St. Joseph's Health Care London and Lawson Scientist
“We know that patients who have injury to their lung and require ventilation have inflammation in the airways of their lungs. Surfactant is a homogeneous layer of lipids and proteins that line the lungs to allow us to breath with minimal effort,” explains Dr. Lewis. “If we gave these patients surfactant as soon as possible after they were put on a mechanical ventilator, it may have some benefit in improving their outcome and getting them off the ventilator sooner.”
Dr. Lewis and his team conducted a clinical trial using pulmonary surfactant with ten critically ill COVID-19 patients and initial results have shown patient safety and efficacy.
Another team of hospital researchers turned to using modified dialysis machines to offer treatment options to those with severe COVID-19 symptoms. Nephrologist at LHSC, Dr. Chris McIntyre, was the first in the world to modify a dialysis device to treat a patient with COVID-19. The device gently removes a patient’s blood, modifies white blood cells, and returns them to fight hyperinflammation.
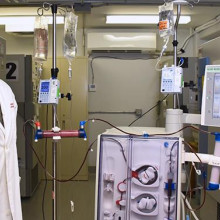
Dr. Chris McIntyre, Nephrologist at LHSC and Lawson Scientist
“It was quick. We went from the initial idea to the approvals, creating the device and appointing the first patient within 40 days,” says Dr. McIntyre who is also a Scientist at Lawson. “After the first 12 patients, we found that those treated with the device needed significantly less drugs to maintain their blood pressure. We were able to deliver the treatment for all the patients – none of the treatments failed and we had no safety issues.”
Dr. McIntyre is now looking into using this form of therapy for chronic dialysis patients to help modify organ injury.