Search
Search
CIHR funding for COVID-19 enables researchers to investigate virus transmission during surgery and pandemic planning
Researchers at Western University and Lawson Health Research Institute continue to make important contributions to help mitigate the spread of COVID-19 and its negative consequences. Two projects in London will address virus transmission during surgery and pandemic planning for COVID-19, thanks to new funding announced by the Government of Canada, through the Canadian Institutes of Health Research (CIHR), along with provincial partners.
Researchers in London received more than $400,000 in funding through this latest round.
“Accelerating high-quality research and real-time evidence is a priority for Canada in its fight against COVID-19. I congratulate the successful teams for their essential work aimed at better preventing, detecting and treating COVID-19 at the individual and population levels,” said Patty Hajdu, Minister of Health in a press release. “Our government believes that it’s through collaboration and data sharing that we will respond efficiently to this global health emergency.”
Virus transmission in surgical smoke
In an effort to perform surgery during the pandemic as effectively and safely as possible, Dr. Leigh Sowerby, Associate Professor at Schulich Medicine & Dentistry and Associate Scientist at Lawson, will be investigating whether or not the virus that causes COVID-19 can be transmitted in surgical smoke. Surgical smoke is the aerosol produced by an essential surgical tool called electrocautery.
“Electrocautery is a ubiquitous tool for surgery, and is known to generate aerosol and smoke. We do not know if the SARS-CoV-2 virus can be transmitted in this plume, and this is important to answer for all surgeons, but in particular, for surgeons working in the respiratory and aerodigestive tract,” said Dr. Sowerby, who is also a head and neck surgeon at London Health Sciences Centre and St. Joseph’s Health Care London. “CIHR funding will allow us to rapidly execute this project. Without this funding, the project would not be possible.”
Dr. Sowerby says the results from this study, whether positive or negative, will have important implications. If positive, it will have a critical and direct impact on ensuring the safety of health care workers performing procedures on patients. Procedures using cautery will continue to require high level protection if the COVID-19 status of the patient is unknown. If negative, it will allow these surgical procedures to continue safely and effectively while conserving critical protective equipment for cases that need it.
The family physician’s role in pandemic plans
Maria Mathews, PhD, Associate Professor at Schulich Medicine & Dentistry, is investigating how the role of family physicians can be better incorporated into pandemic plans. Family physicians play important roles during a pandemic, from detecting potential outbreaks and screening and testing patients to providing care to infected patients and contributing to surge capacity in hospitals.
“During the early stages of the COVID-19 pandemic, family physicians had concerns about roles they were asked to fill for a variety of reasons, including the lack of appropriate personal protective equipment, availability of tests, and concerns about infection risks to other patients and staff in a family practice clinic,” said Mathews.
Mathews will examine the experiences in four regions in Canada – Newfoundland and Labrador, Nova Scotia, Ontario and British Columbia – to identify key roles, supports and best practices. The results will provide government ministries, public health units, and other health organizations with evidence and tools in order to incorporate family physicians in the response to a potential second COVID-19 wave and plan for future pandemics.
Clinical Research
Research at Lawson spans the continuum of life and mirrors the clinical areas of across St. Joseph’s Health Care London. Lawson is involved in all types and phases of clinical trials. Ethical clinical research and participant safety are our utmost priority.
Where does clinical research happen?
Depending on the research being done, it can take place in many different locations, including:
- Doctor’s offices
- Hospitals
- Medical centres
- Community nusing stations
- Academic centres, such as universities and medical schools
- Clinics
- At your home
Frequently Asked Questions
- What is a clinical trial?
- Participating in clinical trials
- Regulations and guidelines
- Considerations for participants and questions to ask
- Clinical Trials Ontario
- It Starts With Me
- Where can I find open clinical trials?
Contact
If you have questions, please call 519-667-6649 or email @email.
Clinical trial will evaluate new therapy for patients with treatment-resistant depression as a result of bipolar disorder
LONDON, ON – Researchers at Lawson Health Research Institute are offering new hope to patients with treatment-resistant depression through participation in a national clinical trial. The study is the first randomized controlled trial to examine the efficacy of a new treatment called magnetic seizure therapy (MST) for patients with treatment-resistant depression as a result of bipolar disorder.
Treatment-resistant depression is a severe form of depression that does not respond to traditional therapies like medication. It is particularly common in patients with depression from bipolar disorder who are left with limited treatment options.
For years electroconvulsive therapy (ECT) has been the gold standard for patients with treatment-resistant depression. ECT uses an electric field to induce a seizure that provides a therapeutic benefit. But while ECT is effective, many patients opt out of treatment due to stigma surrounding the therapy and the potential for cognitive side effects like disorientation and amnesia.
MST has emerged as a promising alternative. MST works in a similar way to ECT but uses a focused magnetic field as opposed to electricity. As a result, MST induces a more focused seizure to reduce the risk of cognitive side effects.
“Magnetic seizure therapy has already been shown as a promising treatment for major depressive disorder or unipolar depression,” explains Dr. Amer Burhan, local site lead, researcher at Lawson and neuropsychiatrist at St. Joseph’s Health Care London. “For the first time, we’re studying how effective the treatment is for depression as a result of bipolar disorder and whether it can reduce the risk of cognitive side effects associated with electroconvulsive therapy.”
The clinical trial is being led by the Centre for Addiction and Mental Health (CAMH) and will also be offered through Lawson and University of British Columbia (UBC) Hospital. Lawson researchers will invite eligible patients with treatment-resistant depression from bipolar disorder to participate in the trial at Parkwood Institute, a part of St. Joseph’s Health Care London.
Eligible patients will be randomized to receive either ECT or MST. Patients will be offered support throughout the study with the goal of improving patient outcomes in both groups. Patient outcomes will be compared to study the efficacy of MST and whether it is associated with reduced cognitive side effects.
MST will be delivered under anesthesia in 12 to 20 sessions. Sessions will last 10 to 15 minutes each with 60 to 90 minutes of recovery time.
“Magnetic seizure therapy holds promise of one day replacing electroconvulsive therapy as the gold standard for treatment-resistant depression,” says Dr. Burhan. If proven as a viable first-line treatment, MST would be very easy to implement in existing ECT clinics. MST would therefore be readily available to patients in need.
“We are on the leading edge of the field of brain stimulation for treatment-resistant depression in collaboration with CAMH and UBC,” says Dr. Burhan. “Our goal is to continue informing the care process through clinically-relevant research that serves patients, medical professionals and the public.”
-30-
Lawson Health Research Institute is one of Canada’s top hospital-based research institutes, tackling the most pressing challenges in health care. As the research institute of London Health Sciences Centre and St. Joseph’s Health Care London, our innovation happens where care is delivered. Lawson research teams are at the leading-edge of science with the goal of improving health and the delivery of care for patients. Working in partnership with Western University, our researchers are encouraged to pursue their curiosity, collaborate often and share their discoveries widely. Research conducted through Lawson makes a difference in the lives of patients, families and communities around the world. To learn more, visit www.lawsonresearch.ca.
Senior Media Relations Consultant
Communications & Public Engagement
T: 519-685-8500 ext. 73502
Celine.zadorsky@lhsc.on.ca
Clinical trial will evaluate new therapy for treatment-resistant depression as a result of bipolar disorder
Treatment-resistant depression is a severe form of illness that does not respond to traditional therapies like medication and counselling. It is particularly common in patients with depression from bipolar disorder who are left with limited treatment options.
“There are some mental illnesses that can become resistant to therapy, similar to how infections, for example, can become resistant to antibiotics,” explains Dr. Amer Burhan, researcher at Lawson Health Research Institute and neuropsychiatrist at St. Joseph’s Health Care London. “Patients with those illnesses need more options.”
Brain stimulation is a field that holds promise for this patient population.
“When people are in a state of depression, research shows their brain networks are not functioning properly,” says Dr. Burhan. “Brain stimulation aims to stimulate neurons in the brain to correct activity and improve patient outcomes.”
Through involvement in a national clinical trial, Dr. Burhan and his research team at Lawson are offering new hope with a treatment called magnetic seizure therapy (MST). The study is the first randomized controlled trial to examine the efficacy of MST for patients with treatment-resistant depression as a result of bipolar disorder.
For years electroconvulsive therapy (ECT), one form of brain stimulation, has been the gold standard for patients with treatment-resistant depression. ECT uses an electric field to induce a seizure that provides a therapeutic benefit by stimulating the brain. But while ECT is effective, many patients opt out of treatment due to stigma surrounding the therapy and its potential for cognitive side effects like disorientation and amnesia.
MST has emerged as a promising alternative. MST works in a similar way to ECT but uses a focused magnetic field as opposed to electricity. As a result, MST induces a more focused seizure to reduce the risk of cognitive side effects.
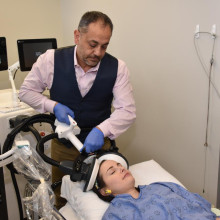
“Magnetic seizure therapy has already been shown as a promising treatment for major depressive disorder or unipolar depression,” explains Dr. Amer Burhan, local site lead for the clinical trial. “For the first time, we’re studying how effective the treatment is for depression as a result of bipolar disorder and whether it can reduce the risk of cognitive side effects associated with electroconvulsive therapy.”
The clinical trial is being led by the Centre for Addiction and Mental Health (CAMH) and will also be offered through Lawson and University of British Columbia (UBC) Hospital. Lawson researchers will invite approximately 30 eligible patients with treatment-resistant depression from bipolar disorder to participate in the trial at Parkwood Institute, a part of St. Joseph’s Health Care London.
Eligible patients will be randomized to receive either ECT or MST. Patients will be offered support throughout the study with the goal of improving patient outcomes in both groups. Patient outcomes will be compared to study the whether MST is effective and whether it is associated with reduced cognitive side effects.
MST will be delivered under anesthesia in 12 to 20 sessions. Sessions will last 10 to 15 minutes each with 60 to 90 minutes of recovery time.
“Magnetic seizure therapy holds promise of one day replacing electroconvulsive therapy as the gold standard for treatment-resistant depression, but we need to learn more about where it fits in our toolbox of potential treatments,” says Dr. Burhan.
If proven as a viable first-line treatment, MST would be very easy to implement in existing ECT clinics. MST would therefore be readily available to patients in need.
“We are on the leading edge of the field of brain stimulation for treatment-resistant depression in collaboration with CAMH and UBC,” says Dr. Burhan. “Our goal is to continue informing the care process through clinically-relevant research that serves patients, medical professionals and the public.”
Those who would like more information about the trial can email @email or @email.