Search
Search
Groundbreaking Alzheimer’s and cancer studies receive $7.2M boost
Lawson Research Institute scientists and partners will focus on molecular imaging and theranostics to potentially transform the detection and treatment of neurodegeneration and cancer.
The quest to advance detection and treatment of Alzheimer’s disease and to personalize cancer care has received a major boost, with $7.2 million in funding to Lawson Research Institute (Lawson) of St. Joseph’s Health Care London (St. Joseph’s) for first-of-its kind research.
Lawson scientists will partner with a broad team of researchers at London Health Sciences Centre Research Institute (LHSCRI), McMaster University, University Health Network and BC Cancer on the ground-breaking studies focused on molecular imaging and theranostics as a potential game-changer in detecting and treating neurodegeneration and cancer, particularly prostate, brain and breast cancer.
Principal investigator Ting-Yim Lee, PhD, Lawson’s Director of PET/CT Research, and his team of investigators were awarded $2 million through the Ontario Research Fund – Research Excellence for the study titled “Improving Cancer and Alzheimer’s Disease Diagnosis and Treatment Through Cutting-edge Molecular Imaging and Theranostics”. Co-Principal Investigator is radiation oncologist Dr. Glenn Bauman at LHSCRI.
Additional funding from private-sector partners and Lawson, as well as from donors through St. Joseph’s Health Care Foundation, brings the total research investment to $7.2 million.
The research has the potential to offer hope for solutions to some of the most prevalent and pernicious diseases affecting Canadians, explains Lee.
“Both research projects are the first of their kind in Canada aimed at advancing how we diagnose and treat Alzheimer’s disease and cancer,” he says. “This collaborative funding initiative will also drive innovation in the exciting field of molecular imaging and theranostics at St. Joseph’s, at the heart of which is St. Joseph’s new, high-sensitivity GE HealthCare Omni Legend 2 PET/CT – the first in Canada.”
The studies encompass the following:
- Alzheimer’s disease: The new PET/CT at St. Joseph’s allows researchers to simultaneously study both blood flow and glucose metabolism in the brain. Both these mechanisms are believed to be contributing factors in the onset of Alzheimer’s. By measuring both at the same time, the research team hopes to uncover early signs that the brain is in trouble and at risk of plaque deposits and toxic proteins that have been linked to the development of Alzheimer’s.
- Cancer: The cancer study will focus on developing theranostic techniques to achieve personalized dosimetry – a method used to determine the exact amount of radiation a patient should receive during treatment, based on their individual characteristics. This maximizes effective treatment while minimizing harm to healthy tissues.
Molecular imaging and theranostics is a rapidly emerging field of medicine that combines ultra-precise scans and theranostics (a term that melds the words therapeutics and diagnostics). Together, they offer a one-two punch by integrating imaging and radiotracers that can identify the location and extent of diseased tissues and selectively destroy the abnormal cells while leaving surrounding healthy cells undamaged. In collaboration with GE HealthCare, St. Joseph’s is developing Canada’s first GE HealthCare Centre of Excellence in Molecular Imaging and Theranostics.
“By bridging the gap between research and clinical practice, we hope to ease the burden on patients and their families, offering more effective and compassionate care”
-Ting-Yim Lee, PhD, Director of PET/CT Research at Lawson Research Institute.
“We are already seeing the impact of novel theranostics for treatment of men with advanced prostate cancer,” says Bauman. “Promising new theranostic approaches are emerging for many cancers and this investment further positions London to be a leader in this area of research.”
In the initial phase of the studies, 100 patients will be recruited from St. Joseph’s Aging Brain and Memory Clinic at Parkwood Institute for the Alzheimer’s study; while 90 patients will be recruited from London Health Sciences Centre’s Verspeeten Family Cancer Centre for cancer studies. There are plans to recruit patients from the collaborating centres once results from the initial phase are confirmed.
“By bridging the gap between research and clinical practice, we hope to ease the burden on patients and their families, offering more effective and compassionate care,” says Lee. “We are deeply grateful for the opportunity to turn our research into real-world solutions that can make a meaningful impact.”
With dozens of 'firsts' in imaging research, “Lawson is a powerhouse of innovation,” adds Michael Kovacs, PhD, Program Lead, Lawson’s Imaging Research Program, and Lead, Cyclotron & PET Radiochemistry Facility. “We're excited to explore how this work could transform care."
Growing Tissues in the Lab
When challenged by surgeons to find better treatments for difficult-to-manage connective tissue diseases, Dr. David O’Gorman gladly accepted.
Dr. O’Gorman is a Molecular Biologist and Lawson Scientist based at St. Joseph’s Hospital, a part of St. Joseph’s Health Care London. His research focuses on understanding normal and abnormal connective tissue repair. He collaborates with researchers and clinicians working in many different disciplines, including those specializing in reconstructive surgery, orthopedics and urology.
Surgical reconstructions can be hampered by a lack of graft tissue, or graft tissue of insufficient quality, making it difficult to achieve optimal outcomes for the patients.
An example is a condition called urethral stricture disease (urethral scarring). This condition occurs in males and typically causes symptoms such as frequent and urgent urination, and slow urinary stream. In extreme cases, it can cause urinary tract infections, permanent bladder dysfunction and renal failure. Recurrence rates after minimally invasive treatments are high, and so many urologists recommend open surgical approaches.
Surgeons can use the patient’s own tissues to reconstruct the urethra after stricture removal. This tissue is normally sourced from the buccal cavity in the mouth but taking large tissue grafts can result in complications. In cases where buccal grafts have been used for previous reconstructions, there may not be enough intact tissue left.
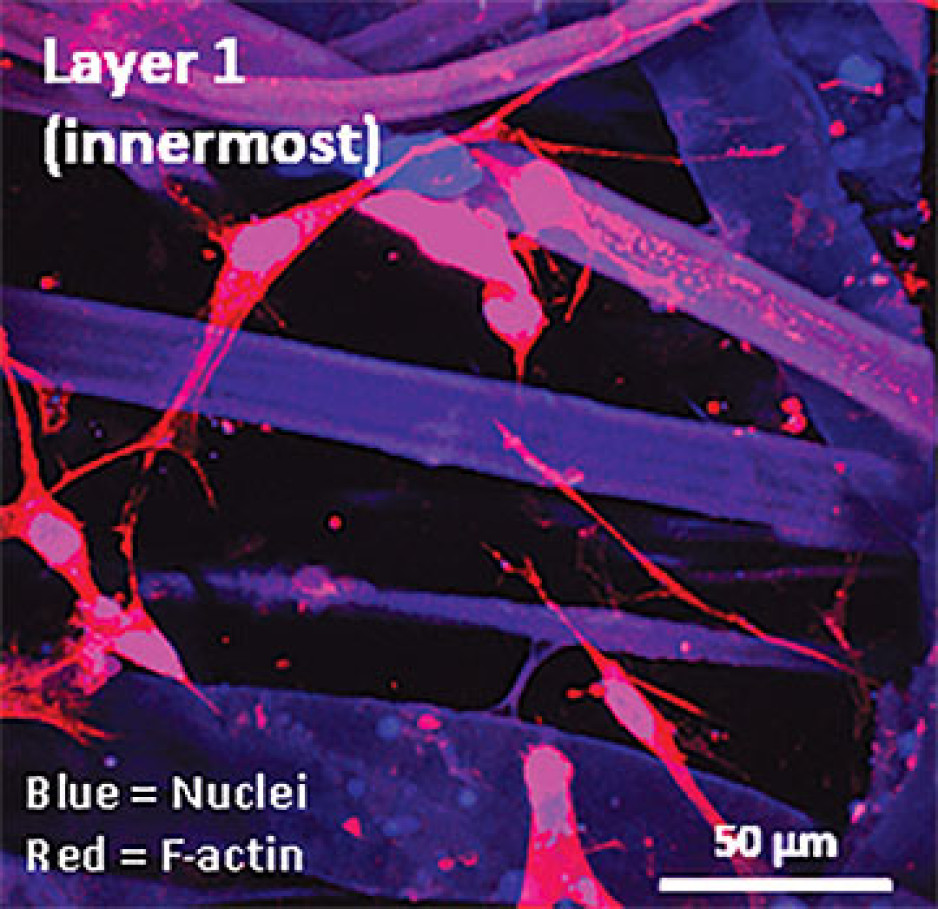
Dr. O’Gorman sees a solution in growing sheets of human buccal tissues in the lab.
“We are currently using buccal graft trimmings as a source of cells, culturing them in a 3D environment and expanding them to create tissues of suitable size, density and elasticity.”
The patient’s own cells are used to generate a tissue graft for urethral reconstruction. While several research groups have developed this approach in the past, few have attempted to translate their models for clinical use.
“Our immediate goal is to provide proof of principle – that we can consistently generate grafts of suitable size and functional characteristics,” explains Dr. O’Gorman, “In the future, we could be providing bioengineered graft tissues for reconstructive surgeries here in London.”
Bioengineered human tissues can also be used as ‘mimetics’ – replications of human tissues – to study diseases, especially those difficult to model using routine laboratory methods.
Instead of a using a growth media or sterile plastic dishes, 3D cell culture is achieved by embedding cells in a matrix of proteins and other molecules normally found in those tissues. In this environment, gene expression and growth is more similar to cells of connective tissues in the body being replicated.
Dupuytren’s disease (or Dupuytren’s Contracture) affects the palmar fascia in the hand, a connective tissue beneath the skin that extends from the base of the palm into the fingers. This disease can be understood as a type of excessive scarring, where normal tissue repair processes have gone awry and dense scar tissue forms, typically causing permanent palm or finger flexion that restricts hand function.
This condition is surprisingly common and may affect more than one million people in Canada. While there are surgical treatment options available, none consistently prevent this disease from recurring in at least a third of patients.
“Due to its high recurrence rate after treatment, Dupuytren’s disease is currently considered incurable. Our challenge is to understand it well enough to develop truly effective treatments,” says Dr. O’Gorman.
Human hands have unique characteristics not found in other species, making animal models impractical. Instead, Dr. O’Gorman’s team extracts cells from the diseased palmar fascia of patients undergoing hand surgeries and bioengineers them into palmar fascia ‘contractures’ in the lab.
“Since the cells from a single palmar fascia sample can be used to grow dozens of little contractures, we can test many different treatments simultaneously to see what works best for each patient.”
This approach may also allow them to determine if Dupuytren’s disease is truly one disease, or a group of similar diseases that cause palm and finger contractures.
“Often, Dupuytren’s disease is clearly heritable, but some individuals have no family history of it and develop apparently sporadic disease,” notes Dr. O’Gorman. “We want to determine if these are truly the same disease at the molecular level.”
Another major cause of abnormal connective tissue repair is infection, and tissue mimetics can play a role here, too. While rare, infections of artificial joint replacements are particularly devastating for patients, as they typically require readmission to hospital to remove the infected joint, weeks of antibiotic-based treatment, and an additional surgery to replace the artificial joint.
In addition to the associated pain and suffering, these procedures are technically challenging and costly to our health care system.
Artificial shoulder joint infections are most frequently caused by the microorganism Cutibacterium acnes (C. acnes). C. acnes infections disrupt normal tissue repair processes after surgery, cause shoulder tissues to die and promote loosening of the artificial joint. These infections are difficult to diagnose, and there is a lack of reproducible
models in which to study them. Dr O’Gorman’s team has set out to create the first human Shoulder-Joint Implant Mimetic (S-JIM) of C. acnes infection.
“While S-JIMs are more complex, they are 3D in vitro cell culture systems designed to mimic human tissues, like those that we use for studying Dupuytren’s disease.”
S-JIMs include layers of artificial human tissue, wrapped around cores of titanium alloy or cobalt chrome, the metals used to create artificial joints. They are co-cultured with C. acnes under low oxygen conditions similar to those that normally occur around artificial shoulder joints.
“We are bioengineering simple 3D cell cultures to more closely mimic the complexity of human tissues, with blood supply, nerves and interactions with other cells.” – Dr. David O’Gorman
Studying the connective tissue layers close to the infection allows researchers to investigate processes that promote infection, such as the formation of a biofilm that harbours and protects the bacteria from the body’s immune system. They are also able to test whether novel treatments can disrupt biofilm formation and increase the effectiveness of antibiotics.
Dr. O’Gorman predicts that in the future, medical researchers will routinely use bioengineered 3D human tissue and organ mimetics to accelerate our understanding of disease.
“The technology is in its infancy, but the potential for using bioengineered human tissues for surgical reconstructions or as disease models is huge. At Lawson, we’re ready to take on health care challenges and build on innovative approaches to improve the quality of life for patients.”
ONLINE EXCLUSIVE: What is 3D cell culture?
Medical researchers have grown human cells in culture media on or in sterile plastic dishes, such as Petri dishes, for more than 50 years.
Some cells, such as blood cells, can survive and grow in suspension, while others like smooth muscle cells need¬ to adhere to a surface to survive and grow. These are often called “2D cell cultures” because the cells grow horizontally across the bottom of the dish.
Some cells derived from connective tissues, such as fibroblasts, are not only adherent, but also very sensitive to the stiffness of their environment (“biomechanically sensitive” cells). Plastic dishes are at least 10,000 times stiffer than most connective tissues, and when biomechanically sensitive cells detect stiff surfaces, they can change the expression of their genes and behave abnormally.
The most common proteins in these tissues - and in the entire human body - are collagens, and one routine 3D cell culture approach is to embed fibroblasts in a collagen gel (gelatin). Fibroblasts in this environment can grow in any direction they choose, and their gene expression is more similar to cells in connective tissues.
These simple 3D cell cultures represent tissue engineering in its most basic form.
“Our challenge is to bioengineer simple 3D cell cultures in the lab to more closely mimic the complexity of human tissues, which have blood supply, nerves and interactions with other cells and tissues that modify their function and ability to heal after injury,” explains Dr. O’Gorman.
Dr. David O’Gorman is a Lawson Scientist and Co-director, Cell and Molecular Biology Laboratory at The Roth | McFarlane Hand and Upper Limb Centre in London, Ontario. He is also an Assistant Professor at Western University.
Gut microbiome may influence how cancer patients respond to oral therapies, study suggests
LONDON, ONTARIO - A new study from Lawson Health Research Institute and Western University illustrates how the gut microbiome interacts with an oral medication in prostate cancer patients, suggesting bacteria in the gut play a role in treatment outcomes. The findings, published in Nature Communications, highlight how the drug abiraterone acetate is metabolized by bacteria in the gut to reduce harmful organisms while promoting those that fight cancer. The team suspects this is one of many examples of how the microbiome influences our response to medications.
“Research is beginning to uncover the ways in which the human microbiome influences cancer development, progression and treatment,” explains Brendan Daisley, a PhD candidate at Western’s Schulich School of Medicine & Dentistry who is conducting research at Lawson. “Our study highlights a key interaction between a cancer drug and the gut microbiome that results in beneficial organisms with anti-cancer properties.”
Traditional prostate cancer therapies are designed to deprive the body of hormones called androgens, which are responsible for prostate cancer growth.
“Unfortunately, traditional androgen deprivation therapies are not always effective,” explains Dr. Joseph Chin, Lawson Associate Scientist, Professor at Schulich Medicine & Dentistry and Urologist at London Health Sciences Centre (LHSC). "In those cases, alternative therapies are explored.”
Abiraterone acetate is a highly effective therapy used in the treatment of prostate cancer that has been resistant to other treatments. While abiraterone acetate also works to reduce androgens in the body, it does so through a different mechanism and, unlike traditional therapies, it is taken orally.
“When drugs are taken orally, they make their way through the intestinal tract where they come into contact with billions of microorganisms,” says Dr. Jeremy Burton, Lawson Scientist, Associate Professor at Schulich Medicine & Dentistry and lead researcher on the study. “While it’s long been a mystery why abiraterone acetate is so effective, our team wondered if the gut microbiome plays a role.”
The team’s study included 68 prostate cancer patients from LHSC, including those being treated with abiraterone acetate and those being treated with traditional androgen deprivation therapies. The research team collected and analyzed patient stool samples, and conducted further experiments in their laboratory at St. Joseph’s Health Care London.
They discovered that patients’ gut microbiomes changed drastically after taking abiraterone acetate. Bacteria in the gut metabolized the drug leading to a significant increase in a bacterium called Akkermansia muciniphila. Referred to as a ‘next-generation probiotic,’ this bacterium’s relevance has recently been explored in several large cancer studies. It’s been shown to facilitate a better response to cancer immunotherapy drugs and it can elicit a wide range of other positive health benefits as well. The increase in Akkermansia muciniphila also led to an increased production of vitamin K2 which is known for anti-cancer properties that can inhibit tumour growth.
The team also observed the impact of androgen depletion on the microbiome. Both abiraterone acetate and traditional androgen deprivation therapies led to a decrease in organisms that utilize androgen.
“These findings clearly demonstrate that the gut microbiome is playing a role in treatment response,” notes Dr. Burton.
The team hopes to further explore drug-microbiome interactions with a goal of harnessing the microbiome to improve treatment outcomes for a variety of diseases. In another study, they are exploring whether fecal microbiota transplants from a healthy donor can change the microbiome of melanoma patients to increase organisms like Akkermansia muciniphila and improve response to immunotherapy. They also plan to study whether analysis of a patient’s microbiome can be used to predict their response to specific therapies.
“While more research is needed, we may one day be able to analyze a patient’s microbiome to determine the best course of treatment or even influence the microbiome to improve outcomes,” says Dr. Burton. “This could lead to a new frontier in personalized medicine.”
The study was made possible through the generous support of The W. Garfield Weston Foundation, St. Joseph’s Health Care Foundation and the Canadian Urologic Oncology Group.
-30-
DOWNLOADABLE MEDIA

Brendan Daisley, a PhD candidate at Western University’s Schulich School of Medicine & Dentistry who is conducting research at Lawson Health Research Institute

Dr. Jeremy Burton (left) and Brendan Daisley (right) are conducting research on microbiome-drug interactions at Lawson Health Research Institute and Western University
Lawson Health Research Institute is one of Canada’s top hospital-based research institutes, tackling the most pressing challenges in health care. As the research institute of London Health Sciences Centre and St. Joseph’s Health Care London, our innovation happens where care is delivered. Lawson research teams are at the leading-edge of science with the goal of improving health and the delivery of care for patients. Working in partnership with Western University, our researchers are encouraged to pursue their curiosity, collaborate often and share their discoveries widely. Research conducted through Lawson makes a difference in the lives of patients, families and communities around the world. To learn more, visit www.lawsonresearch.ca.
Western delivers an academic experience second to none. Since 1878, The Western Experience has combined academic excellence with life-long opportunities for intellectual, social and cultural growth in order to better serve our communities. Our research excellence expands knowledge and drives discovery with real-world application. Western attracts individuals with a broad worldview, seeking to study, influence and lead in the international community.
The Schulich School of Medicine & Dentistry at Western University is one of Canada’s preeminent medical and dental schools. Established in 1881, it was one of the founding schools of Western University and is known for being the birthplace of family medicine in Canada. For more than 130 years, the School has demonstrated a commitment to academic excellence and a passion for scientific discovery.
Senior Media Relations Consultant
Communications & Public Engagement
T: 519-685-8500 ext. 73502
Celine.zadorsky@lhsc.on.ca
Gut microbiome may influence how cancer patients respond to oral therapies, study suggests
A new study from Lawson Health Research Institute and Western University illustrates how the gut microbiome interacts with an oral medication in prostate cancer patients, suggesting bacteria in the gut play a role in treatment outcomes. The findings, published in Nature Communications, highlight how the drug abiraterone acetate is metabolized by bacteria in the gut to reduce harmful organisms while promoting those that fight cancer. The team suspects this is one of many examples of how the microbiome influences our response to medications.
“Research is beginning to uncover the ways in which the human microbiome influences cancer development, progression and treatment,” explains Brendan Daisley, a PhD candidate at Western’s Schulich School of Medicine & Dentistry who is conducting research at Lawson. “Our study highlights a key interaction between a cancer drug and the gut microbiome that results in beneficial organisms with anti-cancer properties.”
Traditional prostate cancer therapies are designed to deprive the body of hormones called androgens, which are responsible for prostate cancer growth.
“Unfortunately, traditional androgen deprivation therapies are not always effective,” explains Dr. Joseph Chin, Lawson Associate Scientist, Professor at Schulich Medicine & Dentistry and Urologist at London Health Sciences Centre (LHSC). "In those cases, alternative therapies are explored.”
Abiraterone acetate is a highly effective therapy used in the treatment of prostate cancer that has been resistant to other treatments. While abiraterone acetate also works to reduce androgens in the body, it does so through a different mechanism and, unlike traditional therapies, it is taken orally.
“When drugs are taken orally, they make their way through the intestinal tract where they come into contact with billions of microorganisms,” says Dr. Jeremy Burton, Lawson Scientist, Associate Professor at Schulich Medicine & Dentistry and lead researcher on the study. “While it’s long been a mystery why abiraterone acetate is so effective, our team wondered if the gut microbiome plays a role.”
Dr. Jeremy Burton (left) and Brendan Daisley (right)
The team’s study included 68 prostate cancer patients from LHSC, including those being treated with abiraterone acetate and those being treated with traditional androgen deprivation therapies. The research team collected and analyzed patient stool samples, and conducted further experiments in their laboratory at St. Joseph’s Health Care London.
They discovered that patients’ gut microbiomes changed drastically after taking abiraterone acetate. Bacteria in the gut metabolized the drug leading to a significant increase in a bacterium called Akkermansia muciniphila. Referred to as a ‘next-generation probiotic,’ this bacterium’s relevance has recently been explored in several large cancer studies. It’s been shown to facilitate a better response to cancer immunotherapy drugs and it can elicit a wide range of other positive health benefits as well. The increase in Akkermansia muciniphila also led to an increased production of vitamin K2 which is known for anti-cancer properties that can inhibit tumour growth.
The team also observed the impact of androgen depletion on the microbiome. Both abiraterone acetate and traditional androgen deprivation therapies led to a decrease in organisms that utilize androgen.
“These findings clearly demonstrate that the gut microbiome is playing a role in treatment response,” notes Dr. Burton.
The team hopes to further explore drug-microbiome interactions with a goal of harnessing the microbiome to improve treatment outcomes for a variety of diseases. In another study, they are exploring whether fecal microbiota transplants from a healthy donor can change the microbiome of melanoma patients to increase organisms like Akkermansia muciniphila and improve response to immunotherapy. They also plan to study whether analysis of a patient’s microbiome can be used to predict their response to specific therapies.
“While more research is needed, we may one day be able to analyze a patient’s microbiome to determine the best course of treatment or even influence the microbiome to improve outcomes,” says Dr. Burton. “This could lead to a new frontier in personalized medicine.”
The study was made possible through the generous support of The W. Garfield Weston Foundation, St. Joseph’s Health Care Foundation and the Canadian Urologic Oncology Group.