Search
Search
Fecal transplants show promise in improving melanoma treatment
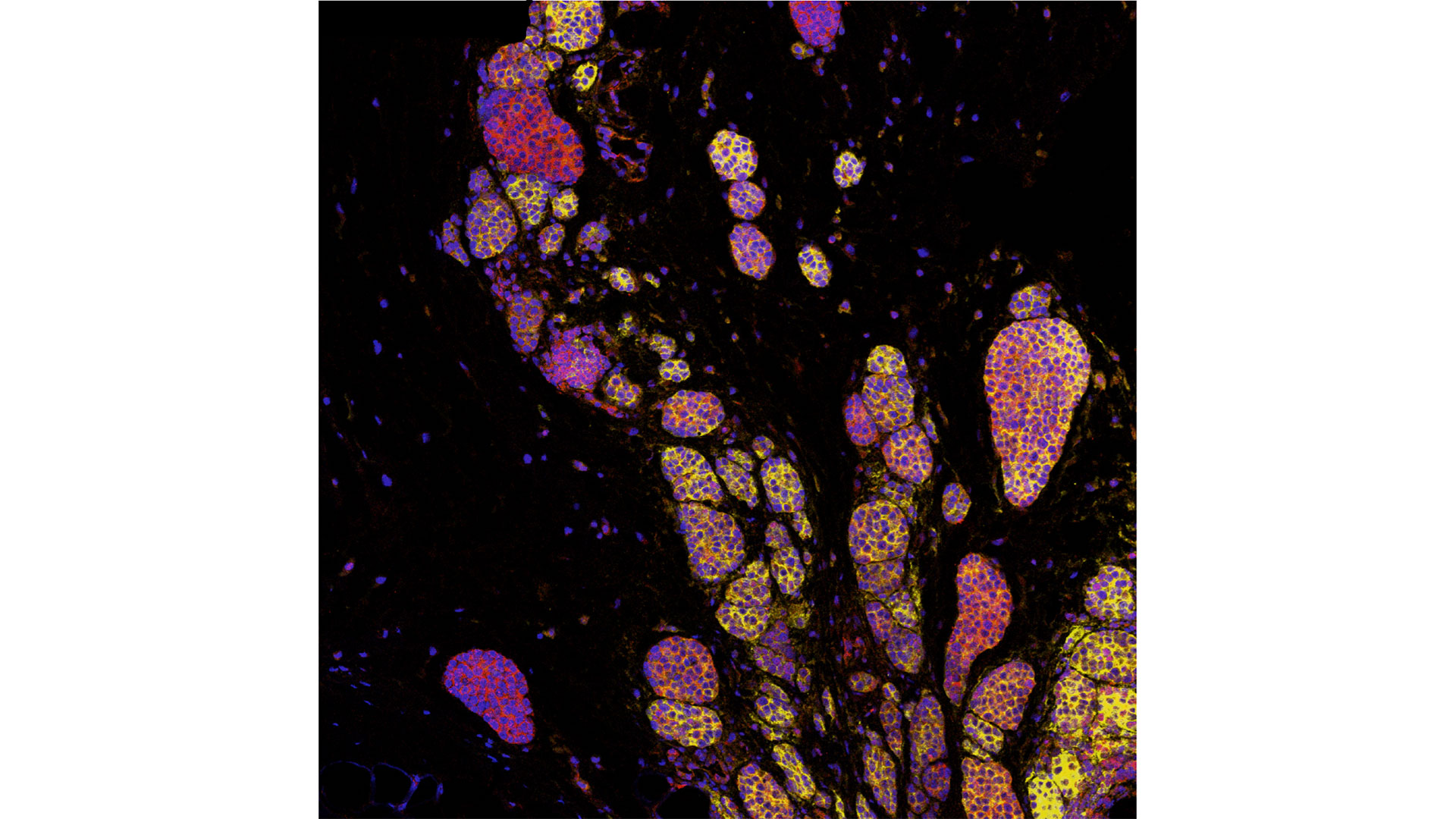
LONDON, ON – In a world-first clinical trial published in the journal Nature Medicine, a multi-centre study from Lawson Health Research Institute, the Centre hospitalier de l’Université de Montréal (CHUM) and the Jewish General Hospital (JGH) has found fecal microbiota transplants (FMT) from healthy donors are safe and show promise in improving response to immunotherapy in patients with advanced melanoma.
Immunotherapy drugs stimulate a person’s immune system to attack and destroy cancer. While they can significantly improve survival outcomes in those with melanoma, they are only effective in 40 to 50 per cent of patients. Preliminary research has suggested that the human microbiome – the diverse collection of microbes in our body – may play a role in whether or not a patient responds.
“In this study, we aimed to improve melanoma patients’ response to immunotherapy by improving the health of their microbiome through fecal transplants,” says Dr. John Lenehan, Medical Oncologist at London Health Sciences Centre’s (LHSC) London Regional Cancer Program (LRCP), Associate Scientist at Lawson and Associate Professor in the Department of Oncology at Western University’s Schulich School of Medicine & Dentistry.
A fecal transplant involves collecting stool from a healthy donor, screening and preparing it in a lab, and transplanting it to the patient. The goal is to transplant the donor’s microbiome so that healthy bacteria will prosper in the patient’s gut.
“The connection between the microbiome, the immune system and cancer treatment is a growing field in science,” explains Dr. Saman Maleki, Scientist at Lawson and LHSC’s LRCP, Assistant Professor in Schulich Medicine’s Departments of Oncology, Pathology and Laboratory Medicine, and Medical Biophysics, and senior investigator on the study. “This study aimed to harness microbes to improve outcomes for patients with melanoma.”
The phase I trial included 20 melanoma patients recruited from LHSC, CHUM and JGH. Patients were administered approximately 40 fecal transplant capsules orally during a single session, one week before they started immunotherapy treatment.
The study found that combining fecal transplants with immunotherapy is safe for patients – which is the primary objective of a phase I trial (also called ‘safety trials’). The study also found 65 per cent of patients who retained the donors’ microbiome had a clinical response to the combination treatment. Five patients experienced adverse events sometimes associated with immunotherapy and had their treatment discontinued.
“We have reached a plateau in treating melanoma with immunotherapy, but the microbiome has the potential to be a paradigm shift,” says Dr. Bertrand Routy, Oncologist and Director of CHUM’s Microbiome Center. “This study puts Canada at the forefront of microbiome research by showing we can safely improve patients’ response to immunotherapy through fecal transplants.”
“These exciting results add to a rapidly growing list of publications suggesting that targeting the microbiome may provide a major advance in the use of immunotherapy for our patients with cancer,” adds Dr. Wilson H. Miller Jr. of the JGH and Professor in the Departments of Medicine and Oncology at McGill University.

The study is unique due to its administration of fecal transplants (from healthy donors) in capsule form to cancer patients – a technique pioneered in London by Dr. Michael Silverman, Lawson Scientist, Chair of Infectious Diseases at Schulich Medicine and Medical Director of the Infectious Disease Care Program at St. Joseph’s Health Care London.
“Our group has been doing fecal transplants for 20 years, initially finding success treating C. difficile infections. This has enabled us to refine our methods and provide an exceptionally high rate of the donor microbes surviving in the recipient’s gut with just a single dose,” says Dr. Silverman. “Our data suggests at least some of the success we are seeing in melanoma patients is related to the efficacy of the capsules."
The team has already started a larger phase II trial involving centres in Ontario and Quebec. Lawson researchers are also studying the potential of fecal transplants in the treatment of other cancers, including renal cell carcinoma, pancreatic cancer and lung cancer, as well as HIV and rheumatoid arthritis.
This research is supported in part through donor funding from London Health Sciences Foundation, Western University, the Lotte and John Hecht Memorial Foundation, the JGH Foundation, Canadian Cancer Society’s Impact Grant program and The Terry Fox Foundation.
-30-
ADDITIONAL DOWNLOADABLE MEDIA


About the CRCHUM
The CHUM Research Centre (CRCHUM) is one of North America’s leading hospital research centres. It strives to improve the health of adults through a continuum of research spanning disciplines such as basic science, clinical research and population health. More than 2,150 people work at the CRCHUM, including nearly 500 researchers and nearly 650 students and postdoctoral fellows. crchum.com
About the Jewish General Hospital
Part of the Integrated Health and Social Services University Network for West-Central Montreal (CIUSSS), the Jewish General Hospital has served patients from Montreal, elsewhere in Quebec, and beyond, since 1934. This McGill University teaching hospital, with approximately 600 beds, is one of the province's largest acute-care hospitals. It admits more than 22,000 patients per year, while handling approximately 578,000 outpatient visits, more than 84,000 emergency visits and more than 3,600 births. Treatment is provided by approximately 800 affiliated doctors, many of whom have teaching appointments at McGill University, as well as more than 300 medical residents per year, together with nursing and a wide range of allied health services.
Lawson Health Research Institute is one of Canada’s top hospital-based research institutes, tackling the most pressing challenges in health care. As the research institute of London Health Sciences Centre and St. Joseph’s Health Care London, our innovation happens where care is delivered. Lawson research teams are at the leading-edge of science with the goal of improving health and the delivery of care for patients. Working in partnership with Western University, our researchers are encouraged to pursue their curiosity, collaborate often and share their discoveries widely. Research conducted through Lawson makes a difference in the lives of patients, families and communities around the world. To learn more, visit www.lawsonresearch.ca.
Communications Consultant & External Relations
Lawson Health Research Institute
T: 519-685-8500 ext. ext. 64059
C: 226-919-4748
@email
Fecal transplants show promise in improving melanoma treatment
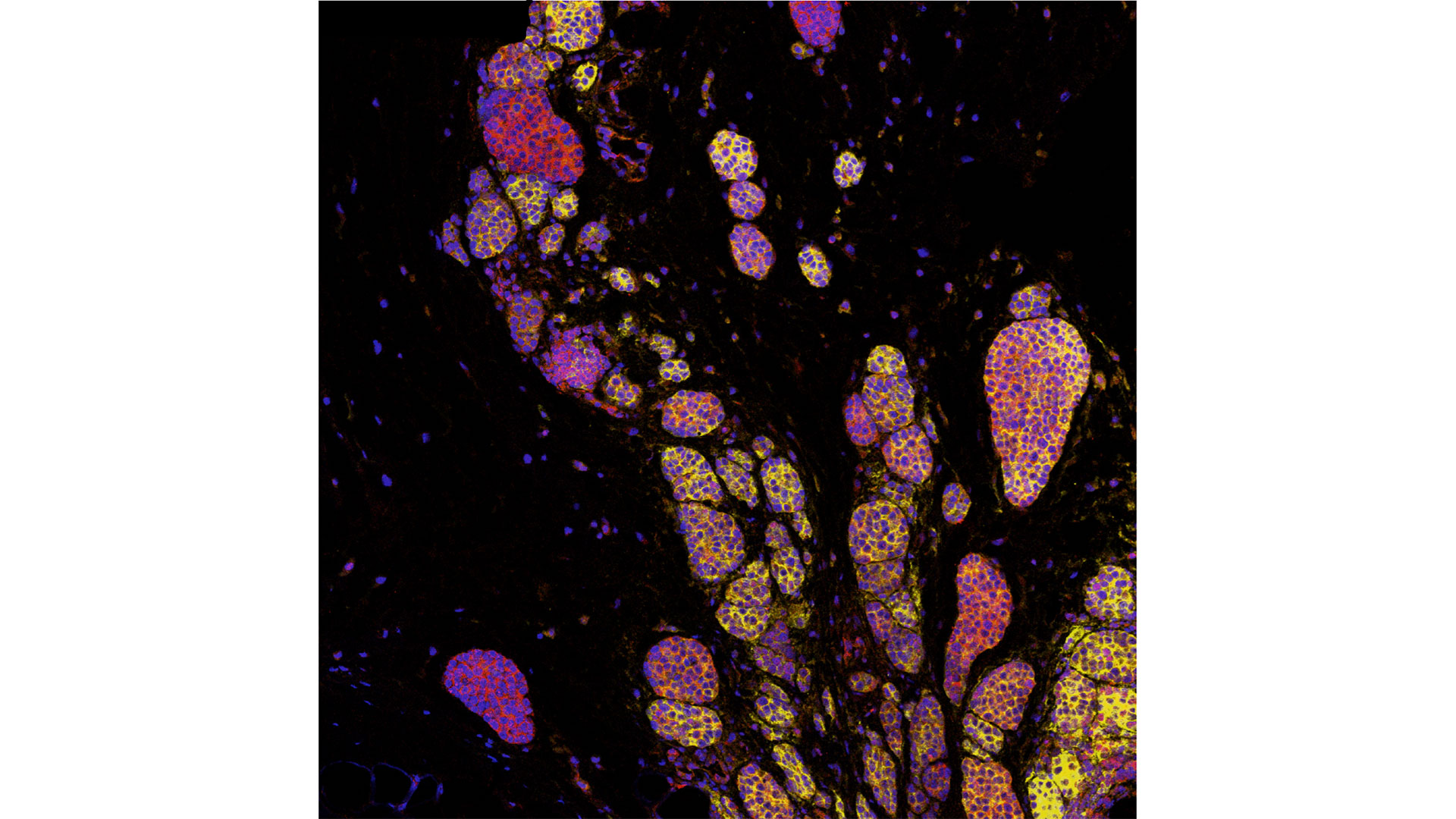
In a world-first clinical trial published in the journal Nature Medicine, a multi-centre study from Lawson Health Research Institute, the Centre hospitalier de l’Université de Montréal (CHUM) and the Jewish General Hospital (JGH) has found fecal microbiota transplants (FMT) from healthy donors are safe and show promise in improving response to immunotherapy in patients with advanced melanoma.
Immunotherapy drugs stimulate a person’s immune system to attack and destroy cancer. While they can significantly improve survival outcomes in those with melanoma, they are only effective in 40 to 50 per cent of patients. Preliminary research has suggested that the human microbiome – the diverse collection of microbes in our body – may play a role in whether or not a patient responds.
“In this study, we aimed to improve melanoma patients’ response to immunotherapy by improving the health of their microbiome through fecal transplants,” says Dr. John Lenehan, Medical Oncologist at London Health Sciences Centre’s (LHSC) London Regional Cancer Program (LRCP), Associate Scientist at Lawson and Associate Professor in the Department of Oncology at Western University’s Schulich School of Medicine & Dentistry.
A fecal transplant involves collecting stool from a healthy donor, screening and preparing it in a lab, and transplanting it to the patient. The goal is to transplant the donor’s microbiome so that healthy bacteria will prosper in the patient’s gut.
“The connection between the microbiome, the immune system and cancer treatment is a growing field in science,” explains Dr. Saman Maleki, Scientist at Lawson and LHSC’s LRCP, Assistant Professor in Schulich Medicine’s Departments of Oncology, Pathology and Laboratory Medicine, and Medical Biophysics, and senior investigator on the study. “This study aimed to harness microbes to improve outcomes for patients with melanoma.”
The phase I trial included 20 melanoma patients recruited from LHSC, CHUM and Jewish General Hospital. Patients were administered approximately 40 fecal transplant capsules orally during a single session, one week before they started immunotherapy treatment.
The study found that combining fecal transplants with immunotherapy is safe for patients – which is the primary objective of a phase I trial (also called ‘safety trials’). The study also found 65 per cent of patients who retained the donors’ microbiome had a clinical response to the combination treatment. Five patients experienced adverse events sometimes associated with immunotherapy and had their treatment discontinued.

“We have reached a plateau in treating melanoma with immunotherapy, but the microbiome has the potential to be a paradigm shift,” says Dr. Bertrand Routy, Oncologist and Director of CHUM’s Microbiome Center. “This study puts Canada at the forefront of microbiome research by showing we can safely improve patients’ response to immunotherapy through fecal transplants.”
“These exciting results add to a rapidly growing list of publications suggesting that targeting the microbiome may provide a major advance in the use of immunotherapy for our patients with cancer,” adds Dr. Wilson H. Miller Jr. of the JGH and Professor in the Departments of Medicine and Oncology at McGill University.
Previous studies looking at patients receiving immunotherapy who do not respond have found many had an unhealthy microbiome, explains Dr. Lenehan.
“There's a portion of people who don't respond or the treatment just doesn't work,” says Dr. Lenehan. “The hope with the fecal transplant is to make more people respond to treatment.”
These results have also led to a closer examination of the role of the microbiome in regulating how the body responds to disease and how the drugs themselves interact with the microbiome.

“The microbes on and in us - and there's actually a huge amount of those – play a critical role, including modulating some of our immune responses,” explains Dr. Jeremy Burton, Research Chair of Human Microbiome and Probiotics, Scientist at Lawson and St. Joseph’s Health Care London and Associate Professor in the Department of Microbiology and Immunology at Schulich Medicine.
The study is unique due to its administration of fecal transplants (from healthy donors) in capsule form to cancer patients – a technique pioneered in London by Dr. Michael Silverman, Lawson Scientist, Chair of Infectious Diseases at Schulich Medicine and Medical Director of the Infectious Disease Care Program at St. Joseph’s Health Care London.
“Our group has been doing fecal transplants for 20 years, initially finding success treating C. difficile infections. This has enabled us to refine our methods and provide an exceptionally high rate of the donor microbes surviving in the recipient’s gut with just a single dose,” says Dr. Silverman. “Our data suggests at least some of the success we are seeing in melanoma patients is related to the efficacy of the capsules."

The team has already started a larger phase II trial involving centres in Ontario and Quebec. Lawson researchers are also studying the potential of fecal transplants in the treatment of other cancers, including renal cell carcinoma, pancreatic cancer and lung cancer, as well as HIV and rheumatoid arthritis.
This work is not possible without poop donors, and there is a critical need for more. Donors must be between the ages of 18 to 50 and reside in the London, Ont. area. To learn more about eligibility and donating, call the 519 646-6100, ext. 61726 or email Dr. Seema Nair Parvathy, Research Scientist, at SeemaNair.Parvathy@sjhc.london.on.ca.
This research is supported in part through donor funding from London Health Sciences Foundation, Western University, the Lotte and John Hecht Memorial Foundation, the JGH Foundation, Canadian Cancer Society’s Impact Grant program and The Terry Fox Foundation.
First clinical guidelines in Canada for pain following spinal cord injury
Researchers at Lawson Health Research Institute are the first in Canada to develop clinical practice guidelines for managing neuropathic pain with patients who have experienced a spinal cord injury (SCI).
Neuropathic pain is complex and chronic, and is the most common complication reported by people following SCI. The research team worked with care providers at Parkwood Institute, part of the St. Joseph’s Health Care London family, and an international panel to address the complex and unique challenges for managing pain during recovery and rehabilitation.
In 2003, Dan Harvey sustained a spinal injury after falling off a trampoline. Using his personal experience, as well as his experiences meeting with newly injured people, Harvey contributed to the development of the new guidelines.
“Neuropathic pain – and pain in general – affects every person with a spinal cord injury very differently. Some people have it, some people don’t. But for those who do have it, it can make rehabilitation and recovery much more difficult,” explains Harvey.
“On top of just learning how to use your body again, you also have to deal with various forms of physical pains, which can make it challenging to mentally adapt to an injury.”
For those with chronic pain, it may be hard to just get out of bed in the morning, feel well enough to leave the house, or even fall asleep. “I have fairly extensive neuropathic pain, so I’m well aware of how difficult it can be to get a full night’s sleep, or show attentiveness at work or at school when it literally feels like your legs are on fire,” says Harvey.

Dan Harvey with Lawson researchers Stacey Guy, Swati Mehta and Dr. Eldon Loh.
Dr. Eldon Loh, Lawson Researcher and Physical Medicine and Rehabilitation Specialist at St. Joseph’s, and his team recognized that pain can be an overlooked part of a spinal cord injury and plays a major factor in the success of rehabilitation. It’s difficult for someone in pain to participate fully in their own recovery, and so long-term disability becomes more likely. Pain is difficult to manage and it often takes multiple approaches to find something that works for each person.
“This is a starting point for us to standardize how we approach pain in the clinic. We have identified gaps and offered recommendations to not only manage the pain, but also ensure that our patients can fully benefit from rehabilitation,” says Dr. Loh.
The results of the three-year process led to recommendations for screening and diagnosis, treatment and models of care. Important clinical considerations accompany each recommendation.
“For those in hospital following an injury, it’s about making sure they can be as independent as possible before discharge. Over time, we want to keep pain levels under control so that they are able to live life to the fullest,” adds Dr. Loh.
The research will inform new tools and resources for care providers and patients.
Harvey believes the guidelines will have a tremendous impact for patients whose pain may have been overlooked. “Pain can be created through many different avenues and the effects can snowball after a person is discharged and sent home. If you don’t check all of the boxes, you might be missing a very important item.”
The new guidelines have been published in the international journal Spinal Cord. The Ontario Neurotrauma Foundation and Rick Hansen Institute provided funding for the research study.
A special thanks to individuals from St. Joseph's who were involved in the project: Steve Orenczuk, Patrick Potter, Keith Sequiera, Lindsey Guilbault, Robert Teasell, Anna Kras-Dupuis, Dalton Wolfe, Alba Casalino and Dwight Moulin.

Additional members of the panel that developed the new clinical practice guidelines.
First Contrast Enhanced Spectral Mammography (CESM) guided biopsy in North America
LONDON, ON – Researchers at Lawson Health Research Institute (Lawson) are the first in North America to perform a breast biopsy guided by Contrast Enhanced Spectral Mammography (CESM).
CESM is a novel diagnostic imaging tool that is able to detect cancerous lesions at a greater rate than standard mammography, and at close rate to MRI. Patients who undergo CESM receive an intravenous iodinated contrast liquid. This liquid acts as a dye that enhances the visibility of certain tissues during a radiographic imaging procedure, such as mammography or x-ray.
Currently, when a suspicious lesion is detected by CESM and not detected by standard mammography or ultrasound, the patient must return later for an MRI biopsy. Wait times for MRI can be lengthy, and the procedure itself is often long and uncomfortable.
CESM guided biopsy offers the ability to perform biopsy of the lesion of concern using mammography, after iodinated contrast injection. The procedure is faster and more accurate, comfortable and cost effective than an MRI biopsy.
The first CESM guided biopsy in North America was performed on June 12, 2020, at St. Joseph’s Health Care London (St. Joseph’s). Dr. Anat Kornecki, Lawson Associate Scientist and Radiologist at St. Joseph’s explains, “this new approach has the potential to provide rapid and accurate access for patients and reduce costs. With CESM biopsy technology we are also able to perform biopsy of lesions that are located in areas that MRI guided biopsy cannot reach.”
“Our initial experience has been very successful, and we hope to see an impact on patient care as well as breast cancer outcomes,” says Dr. Kornecki.
The study will recruit 50 patients who have a suspect finding detected using CESM.
-30-
Lawson Health Research Institute is one of Canada’s top hospital-based research institutes, tackling the most pressing challenges in health care. As the research institute of London Health Sciences Centre and St. Joseph’s Health Care London, our innovation happens where care is delivered. Lawson research teams are at the leading-edge of science with the goal of improving health and the delivery of care for patients. Working in partnership with Western University, our researchers are encouraged to pursue their curiosity, collaborate often and share their discoveries widely. Research conducted through Lawson makes a difference in the lives of patients, families and communities around the world. To learn more, visit www.lawsonresearch.ca.
Senior Media Relations Consultant
Communications & Public Engagement
T: 519-685-8500 ext. 73502
Celine.zadorsky@lhsc.on.ca
First Contrast Enhanced Spectral Mammography guided biopsy in North America
Researchers at Lawson Health Research Institute (Lawson) performed the first breast biopsy guided by Contrast Enhanced Spectral Mammography (CESM) in North America on June 12, 2020.
CESM is a novel diagnostic imaging tool that is able to detect cancerous lesions at a greater rate than standard mammography, and at close rate to MRI. Patients who undergo CESM receive an intravenous iodinated contrast liquid. This liquid acts as a dye that enhances the visibility of certain tissues during a radiographic imaging procedure, such as mammography or x-ray.
Currently, when a suspicious lesion is detected by CESM and not detected by standard mammography or ultrasound, the patient must return later for an MRI biopsy. Wait times for MRI can be lengthy, and the procedure itself is often long and uncomfortable.
CESM guided biopsy offers the ability to perform biopsy of the lesion of concern using mammography, after iodinated contrast injection. The procedure is faster and more accurate, comfortable and cost effective than an MRI biopsy.
The study will recruit 50 patients who have a suspect finding detected using CESM.
Dr. Anat Kornecki, Lawson Associate Scientist and Radiologist at St. Joseph’s Health Care London explains, “this new approach has the potential to provide rapid and accurate access for patients and reduce costs. With CESM biopsy technology we are also able to perform biopsy of lesions that are located in areas that MRI guided biopsy cannot reach.”
“Our initial experience has been very successful, and we hope to see an impact on patient care as well as breast cancer outcomes,” says Dr. Kornecki.
First-in-North-America resource touts health benefit of fermented foods
New network helps consumers, researchers and food industry find and share trusted information about ‘ferment-ceuticals’
London (Ont.) – A one-stop network, the first of its kind in North America, has begun sharing easily digested research, recipes and other resources about the health benefits of fermented foods.
The new Canadian Fermented Foods Initiative (CFFI) launches officially on Nov. 17 with a gathering of research and industry experts from across the country and Europe.
The collaboration helps consumers, researchers, health professionals and food industry share trusted, science-based expertise and information about fermented foods.
Funded by the Weston Family Foundation, the initiative is led by Jeremy Burton, PhD, who heads of one of Canada’s largest microbiome research programs and is Interim Vice President Research at St. Joseph’s Health Care London and Lawson Research Institute. His research leadership is joined by Raylene Reimer, PhD, professor of nutrition at the University of Calgary; and University of Alberta professor Ben Willing, PhD, former Canada Research Chair in Microbiology of Nutrigenomics.
Fermented foods such as sourdough bread, sauerkraut, kimchi and kombucha offer more than just good taste and an economical way to preserve food, Burton says. Large, population-based studies show people who eat fermented foods are generally healthier, with fewer digestive issues and lower risk of chronic diseases.
“How exactly does that work – and why? Well, that’s the big question we’re trying to solve,” Burton says. “One day, I believe, ‘ferment-ceuticals’ will be engrained in our diets and our health vocabulary.”
St. Joseph's is a leader in the field. A paper authored by the team and published this week in Advances in Nutrition represents the most comprehensive synthesis to date of research on fermented foods and human health.
Connor Flynn, a London, Ont., chef, master food preserver and high school teacher whose video recipes are included in the CFFI website, adds, “Fermenting foods is an old practice that’s never fallen out of flavour, but has sometimes fallen out of favour to North Americans. Now it has become popular again.”
To learn more about the CFFI, including fermented food recipes and the chef behind them, head to fermentedfoods.ca.
- 30 -
To arrange interviews with CFFI project lead Jeremy Burton and chef Connor Flynn, who are available Friday Nov. 14 from 7 am – 8 am ET and Nov. 14 from 1 – 4 pm ET, contact:
Deb (Flaherty) Van Brenk, Communication Consultant
St. Joseph’s Health Care London
@email
About St. Joseph’s Health Care London
Renowned for compassionate care, St. Joseph’s Health Care London is a leading academic health care centre in Canada dedicated to helping people live to their fullest by minimizing the effects of injury, disease and disability through excellence in care, teaching and research. Through Lawson Research Institute, our innovation arm, and with collaborative engagement with other health and academic partners, St. Joseph’s has become an international leader in the areas of: chronic disease management; medical imaging; specialized mental health care; rehabilitation and specialized geriatrics; and surgery. St. Joseph’s operates through a wide range of hospital, clinic and long-term and community-based settings, including: St. Joseph’s Hospital; Parkwood Institute; Mount Hope Centre for Long Term Care; and the Southwest Centre for Forensic Mental Health Care.
Funding for unique strategy to prevent homelessness after hospital discharge
In Canada, about 235,000 people experience homelessness each year. The number of homeless people, and the length of time they spend homeless, continues to rise. Homelessness is not a choice and anyone can become homeless.
Although the root cause is poverty, some underlying issues are poor physical or mental health; violence or abuse in the home; lack of employment or income; and, a shortage of affordable housing.
A group of researchers at Lawson Health Research Institute (Lawson), working at both London Health Sciences (LHSC) and St. Joseph’s Health Care London (St. Joseph’s), are committed to tackling the issue of homelessness from within hospital walls, where some patients face the risk of being discharged into homelessness.
“Many of our patients with lived experience of homelessness were saying that their journey started with a hospital discharge,” says Lawson clinician researcher Dr. Cheryl Forchuk. “Often, they were experiencing major transitions in their lives and then experienced a hospital stay. Normally a relatively short visit, they aren’t able to gather the information and make a plan to be able to leave the hospital with somewhere to stay.”
On September 10, Adam Vaughan, Canadian MP and Parliamentary Secretary (Housing and Urban Affairs), on behalf of the Honourable Jean-Yves Duclos, Minister of Families, Children and Social Development, announced that Lawson will receive $223,572 from the Homelessness Partnering Strategy’s (HPS) Innovative Solutions to Homelessness funding stream to support the project “No Fixed Address Version 2 Expansion” research project.

“This is a brilliant approach. It supports an augmented duty of care where hospitals have the means to transfer people into stable settings where they can continue to heal and move towards self-sufficiency,” says Parliamentary Secretary Vaughan.
Taking place at London Health Sciences Centre, this research will further refine the No Fixed Address strategy for reaching and supporting patients during the crucial transitional period when they are being discharged from the hospital and re-integrated into the community.
“Lawson’s expanded No Fixed Address research project is the first evaluation anywhere of a strategy to reduce the number of patients being discharged into homelessness. There is almost no literature on any aspect of this troublesome issue,” explains Dr. Forchuk who is the study’s Principal Investigator. Dr. Forchuk is also the Beryl and Richard Ivey Research Chair in Aging, Mental Health, Rehabilitation and Recovery and Assistant Director at Lawson.

This project is an extension of three previous studies conducted by Dr. Forchuk’s research team, which developed and tested this novel approach. They demonstrated the efficacy, feasibility and cost-effectiveness of using the No Fixed Address strategy in acute and tertiary psychiatric care in the London region, at both LHSC and St. Joseph’s. In the first phase, they found that the interventions used prevented homelessness in 95 per cent of cases.
The researchers are now taking a solution proven to have worked in the mental health units and applying it in selected medical departments at LHSC. Through the study, the services will be available to all patients in those units who are at risk of homelessness. There have already been 17 patients who have accessed this support since the project got underway this summer.
Three community partners from London are supporting implementation of the strategy - Canadian Mental Health Association Middlesex, Ontario Works in the City of London and the Salvation Army’s Housing Stability Bank. They will provide assistance in areas like securing appropriate private-sector housing, provision of income and employment supports, and financial assistance.
“In many ways London, Ontario is the high water mark of solving and tackling homelessness. This community has a lot of be proud of given the way that the municipality is stepping up to the plate and how many different organizations are working together towards a common goal,” says Parliamentary Secretary Vaughan.
This kind of collaboration showcases the important partnership between the Canadian Government, research-intensive hospitals and community organizations to translate innovative solutions from the research stage to the front line of care.
“The hope is that the findings will be even more robust, leading to the development of a best-practice model of hospital discharge that can be adopted throughout Canada. This will reinforce the need for a systemic change in the way hospital discharges occur and ensuring the person is transitioning to a secure housing arrangement,” says Dr. Forchuk.
Learn more about the Government of Canada’s Homelessness Partnering Strategy.
News Coverage
- CBC London - Helping medical patients in London avoid homelessness when they leave hospital
- CTV London - Expanded research hopes to break the cycle of homelessness that psychiatric patients sometimes face when discharged
- Global News, AM 980 - Program that finds housing for homeless patients in hospital gets $223K in federal funding
- London Free Press - 'Brilliant approach' to homelessness gets federal grant