Search
Search
History
Each hospital’s research mission has a rich history. At both hospital organizations, leaders recognized opportunities to leverage in-house experts to conduct research and improve care. However, they also recognized the challenge in supporting these activities without dedicated space and resources.
Through great foresight, our hospitals founded the official research institutes that serve as Lawson's foundation:
- 1983: Supported by Sister Mary Doyle, former Executive Director of St. Joseph's, the Sisters of St. Joseph's establish the hospital's official research institute. LHSC and Upjohn jointly open the Victoria Upjohn Clinical Research Unit at South Street Hospital (formerly Victoria Hospital), focusing on Phase I-III clinical trials.
- 1987: The St. Joseph's research institute is named the Lawson Research Institute (LRI) in honour of London businessman and philanthropist Colonel Tom Lawson and his wife, Miggsie Lawson - close friends of Sister Mary Doyle and major supporters of the research mission.
- 1990: Victoria Hospital takes over the operation of the clinical research unit at South Street, renaming it the Victoria Clinical Trials Centre.
- 1997: The Victoria Clinical Trials Centre is renamed London Health Sciences Centre Research Inc. and becomes a fully incorporated research institute overseeing all hospital-based research within London Health Sciences Centre sites: Victoria Hospital, University Hospital and South Street Hospital.
- 2000: LRI and LHSCRI merge to form a joint venture: Lawson Health Research Institute.
- 2014: Lawson Research Institute (re-)launches as the hospital-based research arm of St. Joseph's with the goal of transforming imagination to innovation to impact; and as LHSCRI is also embedded into LHSC.
Today, partnerships remain strong, allowing researchers to move seamlessly between hospital locations and Western University.
Milestones
Since forming in 2000, Lawson has pioneered breakthroughs across various disciplines of health research and reached several institutional milestones.
- 2019: Lawson led research team is the first in the world to develop a new imaging tool, showed that MRI can be used to measure how the heart uses oxygen.
- 2019: New studies from Lawson and Western University found for the first time that HIV can be transmitted through the sharing of equipment used to prepare drugs before injection and that a simple intervention can destroy the HIV virus, preventing that transmission.
- 2019: In the first genomic analysis of head and neck cancer by smoking status, researchers at Lawson, in collaboration with researchers at the Ontario Institute for Cancer Research and UCLA Cancer Centre, carried out a comprehensive genetic analysis of HPV-negative tumours to better understand the link between smoking and cancer recovery.
- 2019: Lawson scientists develop molecular diagnostic tool to analyze epigenetic patterns, facilitating diagnosis of rare, unknown hereditary disorders. London Health Sciences Centre is the first site in the world to offer this type of testing.
- 2018: Research shows high-dose radiation can improve survival in patients with cancer that has spread to give or less sites. The SABR-COMET study was the first randomized phase II clinical trial of its kind.
- 2018: An international collaborative study between Lawson Health Research Institute, Memorial Sloan Kettering Cancer Center, the Royal Marsden and Epic Sciences is one of the first to demonstrate that a blood test can predict how patients with advanced prostate cancer will respond to specific treatments, leading to improved survival.
- 2018: In collaborative study between Lawson and Stanford University, scientists develop and test a new synthetic surfactant that could lead to improved treatments for lung disease and injury.
- 2018: Scientists use brain MRI to develop first ever method examining young people before they become ill to reliably identify who will develop acute psychosis and who will not.
- 2018: Research team develops clinically-validated, open-source 3D printed stethoscope for areas with limited access to medical supplies.
- 2018: Lawson opens Clinical Research and Chronic Disease Centre (CRCDC) at St. Joseph’s Hospital to tackle chronic disease and improve patient care.
- 2018: Lawson researchers receive $4.4 million to study personalized medicine at LHSC, examining the value of prescribing treatments based on a patient’s genetics.
- 2017: In one of the largest microbiota studies conducted in humans, researchers at Western University, Lawson Health Research Institute and Tianyi Health Science Institute in Zhenjiang, Jiangsu, China have shown a potential link between healthy aging and a healthy gut.
- 2017: Lawson researchers develop transition program to help young adults with type 1 diabetes move from paediatric to adult care.
- 2017: Innovative study brings next-generation genome sequencing to London cancer patients, contributing to province-wide database of genomic and clinical data.
- 2017: Technology developed at Western University and Lawson Health Research Institute can provide a new window into whether or not patients are responding to treatment for advanced ovarian cancer.
- 2017: Dr. Alan Getgood and his team at Western University and Lawson Health Research Institute are the first in Canada to participate in an investigative trial to determine the safety and efficacy of using a patient’s own cartilage cells to repair knee cartilage injuries.
- 2016: Lawson Researchers at Parkwood Institute are the first in Canada to develop clinical practice guidelines for managing neuropathic pain with patients who have experienced a spinal cord injury.
- 2016: Researchers at Lawson are the first in Canada to use a Prostate Specific Membrane Antigen (PSMA) probe in Positron Emissions Tomography (PET) scans to provide improved and highly specific images used for better diagnosis and management of prostate cancer.
- 2015: Lawson scientists, in collaboration with Ceresensa Inc., produce novel PET-transparent MRI head coil, a world first in imaging technology
- 2015: Lawson announces partnership with STEMCELL Technologies for commercialization of tools for Parkinson’s disease research
- 2015: Novare Pharmaceuticals and Lawson announce issuance of a U.S. patent for the composition-of-matter and use of RHAMM-binding peptides with a wide range of potential therapeutic uses. The patent also has claims for the diagnosis and prognosis of cancer, and for prescribing a course of treatment for the diagnosed cancer.
- 2014: Lawson announces licensing agreement with Yabao Pharmaceutical Group in China to develop and test a new life-saving drug to treat sepsis
- 2014: Lawson researchers are part of a Canadian team who have developed a way to produce a key medical isotope, technetium-99m (Tc-99m), using hospital based cyclotrons
- 2013: The Institute for Clinical Evaluative Sciences (ICES) Western opens at Lawson
- 2012: Lawson installs Canada's first PET/MRI at St. Joseph's Hospital
- 2011: Lindros Legacy Research Building officially opens at University Hospital
- 2010: Lawson opens the Cyclotron and PET Radiochemistry facility at St. Joseph's Hospital
- 2009: Lawson receives a record $7 million donation to support the Canadian Research & Development Centre for Probiotics
- 2008: Lawson establishes an experimental anti-thrombolitic clinic to calculate personalized dosage of drugs based on a patient's genetics
- 2007: The first totally endoscopic closed-chest robotic coronary artery bypass surgery on a patient's beating heart is performed at University Hospital
- 2006: Lawson opens the Aging, Rehabilitation & Geriatric Care Research Centre, the first centre of its kind in Canada, at Parkwood Institute
- 2005: Lawson creates the first Ontario Cardiac Rehabilitation Registry
- 2004: Lawson scientists release a three-year study on the effects of the Walkerton water disaster
- 2003: Lawson opens the Victoria Research Laboratories at Victoria Hospital, the first collaboration of its kind in Canada bringing together research from cancer, children's health and vascular biology
- 2002: Lawson installs the first Positron Emission Tomography and Computer Tomography (PET/CT) scanner in Canada at St. Joseph's Hospital
- 2001: St. Joseph's is one of five sites in the world piloting the Diabetes Electronic Management Systems
Hospital data shows longer, costlier stays for patients experiencing homelessness
Nearly 30,000 people last year were homeless when admitted to hospital and/or discharged from hospital, a first-of-its-kind Canadian analysis shows. Almost all of these inpatients were admitted following a visit to an emergency department, and the complexity of their illnesses meant they stayed twice as long as the national average.
“What’s most troubling, based on what we know from other research, is that so many were discharged into the community without stable housing,” says study co-author Dr. Cheryl Forchuk, PhD, Assistant Scientific Director at Lawson Health Research Institute. “We need to see housing as a health intervention, and an integral part of a health strategy,” says Dr. Forchuk, a trailblazer in researching health impact and solutions for people experiencing homelessness.
The new analysis – using a database from the Canadian Institute for Health Information (CIHI) – is the first detailed look at Canadian hospitals’ use of Z59.0, a mandatory record-keeping code intended to identify and improve services for patients experiencing homelessness.
Key findings from this study include:
- Nearly 30,000 patients identified as living without housing were hospitalized across Canada last year.
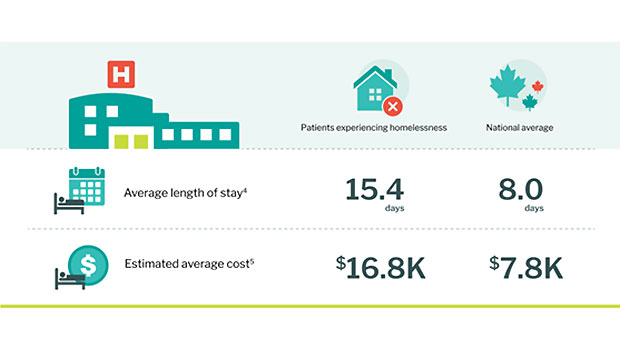
- Average length of stay for people experiencing homelessness was 15.4 days, compared with the national eight-day average.
- About 12 per cent of patients had hospital stays of more than one month.
- Average cost per stay was $16,800, compared with the national average of $7,800.
- Substance use, schizophrenic disorders and cellulitis (a bacterial infection) are the three most common reasons for hospital stays.
- Of these patients, 93 per cent were admitted to hospital after an emergency department visit.

Dr. Forchuk’s contribution to this work was supported through Homelessness Counts, a federally funded Lawson project launched in 2021 to improve understanding of how many people in Canada are experiencing homelessness and who they are.
Dr. Forchuk notes that the longer hospital stays, with more complex care for marginalized populations, can lead to evictions from private apartments or rooming houses.
“In London, we’re in a position to showcase what a community in partnership can do,” Dr. Forchuk notes. “We’ve done a lot of work to prevent discharge into homelessness, including the City of London and other partners prioritizing housing for people who are discharged from hospital.”
The study highlights how housing is intimately connected to health and wellbeing, and the importance of hospitals like London Health Sciences Centre (LHSC) and St. Joseph’s Health Care London (St. Joseph’s) participating in initiatives like the Health & Homelessness Whole of Community Response in London, Ontario.
“As health care providers, we recognize the importance of accurate data for understanding an individual’s care journey across sectors and organizations, especially when addressing homelessness,” says Brad Campbell, Corporate Hospital Administrative Executive, LHSC. “For example, hospital and service utilization data has been essential to understanding emergency department patterns for those living without stable housing in our community, enabling us to improve care for marginalized people through different service delivery models. Through collaborations like our partnership with London Cares, we’ve leveraged data to help individuals in our community access supportive housing and comprehensive 24/7 health and social support services.”
Campbell notes this work aligns to priorities in the Ontario health-care sector by increasing collaboration across sectors and removing barriers to care by enabling inter-agency communication to support increased capacity and access to health-care supports.
“Housing is health care," adds Dr. Forchuk. “Gathering and analyzing this data gives us more tools to find workable solutions to the complex problem of how people experiencing homelessness receive, or don’t receive, the health care they need.
MEDIA CONTACT
Deb (Flaherty) Van Brenk, Communication Consultant
Cell: 226 577-1429 or 519 318-0657
Email: @email
Lawson Health Research Institute is one of Canada’s top hospital-based research institutes, tackling the most pressing challenges in health care. As the research institute of London Health Sciences Centre and St. Joseph’s Health Care London, our innovation happens where care is delivered. Lawson research teams are at the leading-edge of science with the goal of improving health and the delivery of care for patients. Working in partnership with Western University, our researchers are encouraged to pursue their curiosity, collaborate often and share their discoveries widely. Research conducted through Lawson makes a difference in the lives of patients, families and communities around the world. To learn more, visit www.lawsonresearch.ca.
London Health Sciences Centre has been at the forefront of medicine in Canada for 145 years and offers the broadest range of specialized clinical services in Ontario. Building on the traditions of its founding hospitals to provide compassionate care in an academic teaching setting, London Health Sciences Centre is home to Children’s Hospital, University Hospital, Victoria Hospital, the Kidney Care Centre, two family medical centres, and two research institutes – Children’s Health Research Institute and Lawson Health Research Institute. As a leader in medical discovery and health research, London Health Sciences Centre has a history of over 70 international and national firsts and attracts top clinicians and researchers from around the world. As a regional referral centre, London Health Sciences Centre cares for the most medically complex patients including critically injured adults and children in southwestern Ontario and beyond. The hospital’s nearly 15,000 staff, physicians, students and volunteers provide care for more than one million patient visits a year. For more information, visit www.lhsc.on.ca.
Renowned for compassionate care, St. Joseph’s Health Care London is a leading academic health care centre in Canada dedicated to helping people live to their fullest by minimizing the effects of injury, disease and disability through excellence in care, teaching and research. Through partnership with Lawson Health Research Institute and our collaborative engagement with other health care and academic partners, St. Joseph’s has become an international leader in the areas of: chronic disease management; medical imaging; specialized mental health care; rehabilitation and specialized geriatrics; and surgery. St. Joseph’s operates through a wide range of hospital, clinic and long-term and community-based settings, including: St. Joseph’s Hospital; Parkwood Institute; Mount Hope Centre for Long Term Care; and the Southwest Centre for Forensic Mental Health Care. www.sjhc.london.on.ca
Hospital data shows longer, costlier stays for patients experiencing homelessness
Nearly 30,000 people last year were homeless when admitted to hospital and/or discharged from hospital, a first-of-its-kind Canadian analysis shows. Almost all of these inpatients were admitted following a visit to an emergency department, and the complexity of their illnesses meant they stayed twice as long as the national average.
“What’s most troubling, based on what we know from other research, is that so many were discharged into the community without stable housing,” says study co-author Dr. Cheryl Forchuk, PhD, Assistant Scientific Director at Lawson Health Research Institute. “We need to see housing as a health intervention, and an integral part of a health strategy,” says Dr. Forchuk, a trailblazer in researching health impact and solutions for people experiencing homelessness.
The new analysis – using a database from the Canadian Institute for Health Information (CIHI) – is the first detailed look at Canadian hospitals’ use of Z59.0, a mandatory record-keeping code intended to identify and improve services for patients experiencing homelessness.
Key findings from this study include:
- Nearly 30,000 patients identified as living without housing were hospitalized across Canada last year.
- Average length of stay for people experiencing homelessness was 15.4 days, compared with the national eight-day average.
- About 12 per cent of patients had hospital stays of more than one month.
- Average cost per stay was $16,800, compared with the national average of $7,800.
- Substance use, schizophrenic disorders and cellulitis (a bacterial infection) are the three most common reasons for hospital stays.
- Of these patients, 93 per cent were admitted to hospital after an emergency department visit.
Dr. Forchuk’s contribution to this work was supported through Homelessness Counts, a federally funded Lawson project launched in 2021 to improve understanding of how many people in Canada are experiencing homelessness and who they are.
Dr. Forchuk notes that the longer hospital stays, with more complex care for marginalized populations, can lead to evictions from private apartments or rooming houses.
“In London, we’re in a position to showcase what a community in partnership can do,” Dr. Forchuk notes. “We’ve done a lot of work to prevent discharge into homelessness, including the City of London and other partners prioritizing housing for people who are discharged from hospital.”
The study highlights how housing is intimately connected to health and wellbeing, and the importance of hospitals like London Health Sciences Centre (LHSC) and St. Joseph’s Health Care London (St. Joseph’s) participating in initiatives like the Health & Homelessness Whole of Community Response in London, Ontario.
“As health care providers, we recognize the importance of accurate data for understanding an individual’s care journey across sectors and organizations, especially when addressing homelessness,” says Brad Campbell, Corporate Hospital Administrative Executive, LHSC. “For example, hospital and service utilization data has been essential to understanding emergency department patterns for those living without stable housing in our community, enabling us to improve care for marginalized people through different service delivery models. Through collaborations like our partnership with London Cares, we’ve leveraged data to help individuals in our community access supportive housing and comprehensive 24/7 health and social support services.”
Campbell notes this work aligns to priorities in the Ontario health-care sector by increasing collaboration across sectors and removing barriers to care by enabling inter-agency communication to support increased capacity and access to health-care supports.
“Housing is health care," adds Dr. Forchuk. “Gathering and analyzing this data gives us more tools to find workable solutions to the complex problem of how people experiencing homelessness receive, or don’t receive, the health care they need.
Media Contact
Deb (Flaherty) Van Brenk, Communication Consultant
Cell: 226 577-1429 or 519 318-0657
Email: @email
Hospital-based research in London ranked in the top 10 for Canada
Lawson Health Research Institute is ranked eighth in the country according to the 2018 edition of “Canada’s Top 40 Research Hospitals List” by Re$earch Infosource. This strong position has been maintained by Lawson for the past five years and also keeps the institute within the top five institutions in Ontario.
The research institute of London Health Sciences Centre (LHSC) and St. Joseph’s Health Care London (St. Joseph’s), Lawson has also maintained the top ranking for research intensity among the large tier institutions, with $616,300 of research spending per researcher.
“As a hospital-based research institute, our innovation happens where care is delivered,” says Dr. David Hill, Lawson Scientific Director. “Every day, teams of researchers are working directly with clinicians and patients to improve treatments, or create entirely new ones. They find innovative methods of delivering services that drive efficiency and reduce costs.”
The top 40 list analyzes hospital-based research institutes from across the country on several metrics, including total research income from the previous fiscal year. The ranking looks at funds received from all sources, including both internal and external, to support research at the organization. According to the report, Lawson received $123,255 million in research income in 2017, which was a 0.8 per cent drop from the previous fiscal year.
Dr. David Hill advocates for increased scientific funding nationally. “We held our position despite the modest decrease in funding. Canada as a whole requires significant investment in scientific discovery to increase the well-being of Canadians and build a robust economy.”
This year, a special spotlight on intellectual property (IP) is showcasing the top Canadian organizations – universities, corporations, hospitals and government departments/agencies – patenting at the US Patent and Trademark Office.
Lawson, as the research institute of LHSC and St. Joseph’s, is featured in the top 10 list for Hospital Patent Leaders as measured by ownership of patents granted between 2013-2017. Lawson has ranked in the sixth spot with 13 patents owned.
Commercialization opportunities are managed through WORLDiscoveries®, the business development arm of London’s extensive research network. Born out of a partnership between Lawson, Robarts Research Institute and Western University, WORLDiscoveries® draws upon a mix of industry connections, sector-specific market knowledge, and business development expertise to help researchers and local inventors commercialize their discoveries through licensing and new company spin-offs.
“We support technology development and licensing agreements by taking local knowledge and discoveries to industry partners worldwide,” explains Dr. Hill. “Research-intensive hospitals are improving health care, creating jobs and contributing to the country’s growing knowledge economy.”
Featured Innovations
A Stroke of Genius

Based at St. Joseph’s Hospital in London, Dr. Ting-Yim Lee specializes in computed tomography (CT) imaging, a type of x-ray technology that captures images of slices of the body. As a young scientist, he dreamed of using CT imaging to measure how blood flows in the human body. The idea was to develop software that could be installed on existing CT scanners to make quick, easy work of a very complex algorithm. If a patient came to the emergency room suffering from a stroke, it would allow the doctor to quickly analyze and address the damage.
Thanks to decades of public and private sector support, Dr. Lee’s idea has evolved from concept to prototype to clinically-approved product. Through a licensing deal with GE Healthcare, his software is now installed on 70 per cent of the company’s new CT scanners on the market. It’s currently in use in more than 8,000 hospital imaging departments around the world.
Stroke is a situation where every minute of delay in treatment has grave consequences on the recovery of the patient, and this software allows physicians to quickly decide on the best treatment for the patient. Dr. Lee is extending his technology to measure blood flow in whole organs, including predicting and monitoring how cancer and heart attacks respond to treatment. The royalties from the licenses enabled Lawson to install Canada’s first PET/CT scanner to complement CT Perfusion with metabolic information from PET scanning.
Novel discovery in the field of Parkinson’s Disease

In addition to a busy neurosurgery practice, Dr. Matthew Hebb maintains a highly productive research program. Dr. Hebb is creating tools to advance Parkinson’s Disease research and therapeutics across the globe. Parkinson’s Disease is characterized by progressive neurological impairment caused by the death of cells in the nervous system. Dr. Hebb’s team provided a novel description of brain-derived progenitor cells (BDPCs) that could protect and stimulate re-growth of disease-affected neurons. This discovery may offer critical insight into the disease process and provide a new personalized source of brain-derived cells for delivering therapy back into the same individual.
By using a patient’s own BDPCs, Dr. Hebb hopes to slow or halt disease progression and stimulate regeneration of damaged brain circuitry. BDPCs may further advance drug, genetic and functional screening across broad patient populations. This work also resulted in a patent and partnership with STEMCELL Technologies to develop innovative research tools for Parkinson’s Disease and other incurable neurological diseases.
Computer assisted surgical techniques and technologies
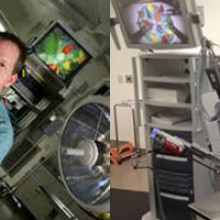
Dr. Christopher Schlachta is Medical Director of CSTAR, the Canadian Surgical Technologies & Advanced Robotics, at LHSC. His current research interests are focused on development of computer-assisted surgical techniques and technologies to enhance care and training. Along with his team, he has demonstrated how computer-assisted technologies in the operating room can enhance communication among surgeons and trainees to produce better outcomes for patients. He is currently partnering with industry to commercialize operating room technology developed with engineers at CSTAR.
His Wireless Hands-free Surgical Pointer system incorporates infrared and inertial tracking technologies to address the need for hands-free pointing during minimally invasive surgery. The combination of these technologies allows for optimal movement of the pointer and excellent accuracy while the user is located at a realistic distance from the surgical monitor.
Smart tech, smart treatment for movement disorders
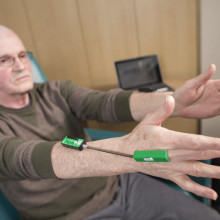
Dr. Mandar Jog operates the London Movement Disorders Centre and has driven the development of TremorTek, a wearable sensor technology that has already successfully treated hundreds of research patients who suffer from tremors in their arms and hands. These tremors, typically caused by Parkinson’s disease or essential tremor, are a common movement disorder symptom yet there is no effective treatment. Neurotoxin therapy has been identified as a possible treatment for tremors; however, an injection in the wrong place or at the wrong dose can cause negative side effects. Everyone experiences tremors in different ways – the location and strength of the movements, and how often they occur varies widely.
Using commercially available sensor technology, Dr. Jog and his team were able to isolate independent muscle movements. They created a system that matched the muscle activity pinpointed by the sensors with the correct amount of toxin to administer. This kinematic technology can be applied to the pre-treatment assessment of patients and the information generated can guide the placement of botulinum toxin. The technology has been taken by a spinoff company MDDT Inc. that has been working with numerous stakeholders interested in its applications.
Imaging “hidden” regions of the heart
After suffering a heart attack, some patients develop a microvascular obstruction, an area of the injured heart with extremely poor blood flow. These patients are at an increased risk of developing heart failure in the future.
Medical imaging technologies such as magnetic resonance imaging (MRI) and positron emission tomography (PET) can be used to study the remodeling process after a heart attack that can lead to a microvascular obstruction. However, poor blood flow makes it difficult to get contrast agents into the obstruction. Contrast agents are used in medical imaging to show contrast between different types of tissue, such as damaged and healthy tissue.
Benjamin Wilk, a PhD candidate at Lawson Health Research Institute and Western University’s Schulich School of Medicine & Dentistry, will investigate whether a hybrid PET/MRI system and a new method of administering contrast agents can allow researchers to image microvascular obstructions and study these “hidden” regions in the heart.
Contrast agents are usually injected as a bolus, meaning the entire injection is administered immediately. In this study, participants will instead receive a constant infusion of an MRI contrast agent and PET tracer, which means the injection will be delivered over the course of an hour. The MRI contrast agent they are using is sensitive to blood flow and scar tissue, and the PET tracer is sensitive to inflammatory cells.
This will allow researchers to study the anatomy, blood flow and inflammatory processes in microvascular obstructions a week after heart attack. Participants will then be imaged again after six weeks to study the long-term effects on heart function.
“Studying the heart after a heart attack using novel contrast agent injection strategies with simultaneous PET/MRI could provide crucial information for treatment planning, helping us reduce the number of people affected by heart failure in the future,” says Wilk. “This project could also lead to further research into finding better ways to administer PET tracers and MRI contrast agents. These methods could be applied to different diseases as well.”
Wilk received a Lawson Internal Research Fund (IRF) Studentship to conduct the study, which will be supervised by Dr. Frank Prato, Assistant Director, Lawson and leader of the Lawson Imaging research program at St. Joseph’s Health Care London.
“Lawson's IRF is valuable for students for many reasons. It not only allows us to conduct further research, it also enriches our experience by giving us opportunities to write grants and attend conferences,” adds Wilk.
The IRF is designed to provide Lawson scientists the opportunity to obtain start-up funds for new projects with the potential to obtain larger funding, be published in a high-impact journal, or provide a clinical benefit to patients. Funding is provided by the clinical departments of London Health Sciences Centre and St. Joseph’s Health Care London, as well as the hospital foundations (London Health Sciences Foundation and St. Joseph’s Health Care Foundation).
Imaging the microbiome
Normally samples of bacteria must be removed from their microbiome environment for analysis, which can lead to changes in their metabolic activity and other behaviors, hindering our ability to accurately study the gut or urogenital microbiome.
“This could be avoided if we are able to observe the bacteria in the body using Magnetic Resonance Imaging (MRI),” says Sarah Donnelly, MSc student at Lawson Health Research Institute and the Department of Microbiology and Immunology and collaborative Molecular Imaging program at Western University’s Schulich School of Medicine & Dentistry.
She is investigating the possibility of using magnetically-labelled bacteria with MRI to more directly study microbial interactions in urological and other conditions.
“The hope is that in the future we can use imaging technologies to visualize aspects of the microbiome in its healthy state compared to diseased states to see the early signs of disease and take preventative measures or allow for early intervention,” she says.
Donnelly has received a Lawson Internal Research Fund (IRF) Studentship to conduct the study, which will be supervised by Dr. Jeremy Burton, scientist in Lawson’s Human Microbiome and Probiotics research program at St. Joseph’s Health Care London (St. Joseph’s) and appointed to the Departments of Surgery and Microbiology & Immunology at Schulich Medicine & Dentistry; and Dr. Donna Goldhawk, scientist in Lawson’s Imaging research program at St. Joseph’s and assistant professor in the Department of Medical Biophysics at Schulich Medicine & Dentistry.
Escherichia coli (E. coli) are a common bacterium found in the human gut microbiome and frequently cause non-intestinal conditions like urinary tract infections. The researchers will program E. coli to express an iron uptake gene, magA. This gene is taken from another type of bacteria called magnetotactic because of their response to Earth’s magnetic field. The researchers will study whether the increase in iron uptake caused by magA expression will allow MRI to detect the magnetic signal more clearly than it would in images of untransformed E.coli. This would make it possible to see the bacteria’s behavior in living subjects without removing the bacteria cells from the microbiome environment.
They will then use this technique to study how magA labelled bacteria affect biofilm on medical devices. A biofilm is a structure produced when certain bacteria adhere to a surface and then stick together.
They will also analyze how lithotripsy affects the bacteria’s spatial distribution and interactions in three-dimensional models of kidney stones. Lithotripsy uses shockwaves to break up kidney stones into smaller pieces that are able to pass naturally out of the body. However, these shockwaves not only affect kidney stones. The waves are sent throughout the tissue, and the bacteria living on these tissues may also be affected.
“While lithotripsy is effective in treating kidney stones, we don’t know the side effects of lithotripsy on the microbiome. The shockwaves could disturb the bacteria, potentially leading to diseases caused by an imbalance between helpful and harmful bacteria,” says Donnelly.
These laboratory models will allow the researchers to perform studies in vivo (in animal models) in the future.
“Health research is very important for the development of new technologies and treatments but it is often difficult to secure funding. The IRF program allows students to pursue research that would not otherwise be possible,” explains Donnelly.
The IRF is designed to provide Lawson scientists and students the opportunity to obtain start-up funds for new projects with the potential to obtain larger funding, be published in a high-impact journal, or provide a clinical benefit to patients. Funding is provided by the clinical departments of London Health Sciences Centre and St. Joseph’s Health Care London, as well as the hospital foundations (London Health Sciences Foundation and St. Joseph’s Health Care Foundation).
Improved imaging for prostate cancer
Prostate cancer is the most commonly diagnosed cancer among Canadian men. However, with improved testing and better treatment options, the number of deaths from the disease has been declining over the last several years.
Scientists at Lawson Health Research Institute are working to continue this trend by testing improved prostate cancer imaging using a new molecule. Known as a Prostate Specific Membrane Antigen (PSMA) probe, the new molecule is used in Positron Emissions Tomography (PET) scans. The probe targets PSMA, a unique molecule on prostate cancer cells, to provide highly specific images for better diagnosis and management of patient disease.
PET probes are used in imaging to correctly diagnose cancer. The probes are injected into a patient where they spread to identify sites of disease. The most common PET probes are suitable for many types of cancer, but are not as sensitive in identifying prostate cancer. PSMA probes provide higher accuracy by targeting PSMA molecules, which are highly over-expressed on prostate cancer cells.
PSMA probes are gaining popularity across the globe. This specific probe is a molecule called 18F-DCFPyL and was developed by Dr. Martin Pomper at the John Hopkins Hospital in Baltimore. Dr. Pomper, also a Scientific Advisor to Lawson’s prostate imaging team, worked in collaboration with Canada’s Centre for Probe Development and Commercialization (CPDC) to bring the probe to our nation.
Lawson’s Canadian Institutes of Health Research (CIHR) Team in Image Guidance for Prostate Cancer gained early access to the PSMA probe due to a history of close collaboration with Dr. Pomper and the CPDC. Marking the first time a PSMA probe has been used in Canada, the team captured PET/MRI and PET/CT images from a 64-year-old prostate cancer patient on March 18, 2016 at St. Joseph’s Hospital.
“This is a tremendous step forward in the management of prostate cancer,” said Dr. Glenn Bauman, a Lawson scientist and Radiation Oncologist at London Health Sciences Centre. “PSMA probes have the potential to provide increased accuracy and detection which leads to better, personalized treatment.”
Lawson plans to study the probe with an additional 20 men over the next two years as part of an ongoing clinical trial funded by the Ontario Institute for Cancer Research (OICR). Lawson scientists are working with researchers across Ontario to develop other clinical trial protocols that will use 18F-DCFPyL to measure responses to drug treatments and to evaluate men with suspected recurrence of prostate cancer after radiotherapy.
“The goal of these studies it to establish the value of PSMA probes, particularly18F-DCFPyL, and provide evidence to support the use of these probes in routine clinical care,” said Dr. Bauman.
Donor funding through London Health Sciences Foundation was one catalyst in this research, providing initial funding to hire Research Associate, Catherine Hildebrand, who set up citywide cancer imaging workshops and helped the team prepare successful grant applications to secure key funding from CIHR and OICR.
Improving recovery and rehabilitation for patients with mental illness
Over 130 hospital-based clinical, administrative and research staff members, persons with lived experience of mental illness, family caregivers, peer and community supporters, and staff from local community mental health agencies attended the 18th Annual Mental Health Research & Innovation Half Day on November 1, 2017. The event provided an opportunity to learn more about mental health research at Parkwood Institute and the Southwest Centre for Forensic Mental Health Care (Southwest Centre), part of the St. Joseph’s Health Care London (St. Joseph’s) family.
“This year’s Mental Health Research and Innovation Half Day was one of the best attended in our history of hosting the event. We had a very diverse and engaged audience with great energy and a lot of enthusiasm,” says Dr. Arlene MacDougall, Director of Research and Innovation for mental health care at St. Joseph’s and Lawson Health Research Institute (Lawson).
Exciting recent projects were showcased though talks highlighting excellence in recovery and rehabilitation research, the theme of this year’s event; poster presentations; the 13th Annual Tony Cerenzia Research Lecture delivered by Dr. Sean Kidd; and interactive workshop sessions.

“We chose ‘recovery and rehabilitation’ as the theme for the event because it is so important in our clinical care and research programs to have this focus. We need to prioritize the development, implementation and evaluation of practices and interventions that foster recovery of the whole person experiencing mental illness, which includes their psychological, social and other needs that go beyond traditional notions of healthcare,” Dr. MacDougall adds.
13th Annual Tony Cerenzia Research Lecture
Guest speaker Dr. Kidd’s talk – “From clinical trials to the clinic: A story about making Cognitive Adaptation Training for schizophrenia more accessible” – focused on how to implement interventions proven in clinical trials. Dr. Kidd is a clinical psychologist, senior scientist and psychology division chief at the Centre for Addiction and Mental Health (CAMH) in Toronto. He is also an associate professor in the Department of Psychiatry at the University of Toronto.

Above: Dr. Sean Kidd's lecture focused on implementing interventions proven in clinical trials.
Workshop Sessions
Following Dr. Kidd’s lecture, attendees had the opportunity to participate in one of six workshops on a variety of topics related to recovery and rehabilitation focused mental health research:
“Implementing Interventions: A facilitated conversation attending to evidence, strategy, and recovery oriented care”
Led by Dr. Sean Kidd
Participants shared successful strategies for implementing novel approaches to care and discussed the challenges involved. They also looked at ways to leverage technology and education materials.
“Spirituality in Mental Health Care: Practically Supporting Recovery and Wellness”
Led by Stephen Yeo, Lawson allied scientist and chaplain, Southwest Centre; and Dr. Clark Heard and Jared Scott, Lawson associate scientists and occupational therapists, Southwest Centre
This workshop focused on the practical application of spirituality within the clinical setting, including the use of labyrinths, which contribute to recovery by promoting spiritual self-care, insight development and personal meaning-making reflection. Attendees had the opportunity to participate in a labyrinth walk and a related spiritual reflection. Read more about the labyrinths at Parkwood Institute and the Southwest Centre or watch the following video featuring highlights from the workshop:
“Indigenous Men’s Health and Wellbeing: Connection with Culture as a Rehabilitation and Recovery Tool”
Led by Bill Hill, social worker, Parkwood Institute and Dr. Vicki Smye, associate professor, director of nursing at Western University
Through the sharing of practitioner experience and Indigenous men’s voices, the workshop focused on understanding the powerful links between connection to culture and mental health and well-being (pictured below).

“Engaging Service Users and their Families in Research”
Led by Dr. Cheryl Forchuk, The Beryl and Richard Ivey Research Chair in Aging, Mental Health, Rehabilitation and Recovery, Lawson; and Deborrah Sherman, executive director, Ontario Peer Development Initiative
Participants in this workshop discussed the benefits of patient and family involvement in mental health research and identified strategies to support patient and family engagement (pictured below).

“Innovation in Mental Health Care: Moving Ideas to Impact”
Led by Kaitlin Saxton, research and innovation facilitator, Parkwood Institute; and Lisa Bitcola, centre manager of projects and operations, Ivey International Centre for Health Innovation
This workshop focused on how innovation relates to research and quality improvement initiatives within St. Joseph’s Mental Health Care facilities. Participants discussed innovative approaches that could be implemented within their own clinical practice, research and quality improvement initiatives (pictured below).

“My Professional Practice: Where's the Research?”
Led by Amanda Thibeault, director, professional practice, St. Joseph’s
In this session, participants discussed how they can incorporate research into their clinical practice (pictured below).

Improving surgery for wrist arthritis
Wrist arthritis can cause debilitating pain, weakness and decreased range of motion. When patients are first diagnosed, the condition can often be managed with activity modification and pain medication. However, as symptoms progress, patients eventually require surgery.
Surgeons typically perform a procedure called four-corner fusion to preserve wrist motion and provide pain relief. This surgery involves removing one of the carpal bones and fusing four of the remaining carpal bones. Although this procedure is one of the most common treatments for wrist arthritis, it is not known how the position of the fusion of the wrist bones affects range of motion and joint contact.
Lawson associate scientist Dr. Nina Suh is leading a study with the goal of improving the surgical technique for four-corner fusion to maximize wrist function and symptom relief, and delay wrist arthritis progression.
Dr. Suh and her team will use a customized active-motion wrist simulator to create different carpal bone fusion positions. They will then assess how these positions affect wrist motion and joint contact area.
“We hope this research will lead to new surgical techniques that will help us to more effectively manage wrist arthritis with four-corner fusion,” says Dr. Suh, who is also an orthopaedic surgeon at the Roth McFarlane Hand and Upper Limb Centre (HULC) at St. Joseph’s Health Care London and an assistant professor at Western University’s Schulich School of Medicine & Dentistry. “The project will also advance our understanding of wrist biomechanics, providing a foundation for the development of enhanced patient-specific surgical tools, such as custom wrist fusion devices and implants.”
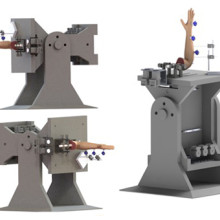
Image of the customized active-motion wrist simulator Dr. Nina Suh and her team are using to create different carpal bone fusion positions. They will then assess how these positions affect wrist motion and joint contact area.
The study is being funded through the Lawson Internal Research Fund (IRF), designed to allow scientists the opportunity to obtain start-up funds for new projects with exciting potential.
“The IRF program is valuable for scientists as external funding sources routinely require preliminary data to strengthen applications,” says Dr. Suh. “Particularly for new scientists such as myself, these grants provide seed funding that allows us to demonstrate the validity of our methodology and the clinical usefulness of our results.”
The IRF is designed to provide Lawson scientists the opportunity to obtain start-up funds for new projects with the potential to obtain larger funding, be published in a high-impact journal, or provide a clinical benefit to patients. Funding is provided by the clinical departments of London Health Sciences Centre and St. Joseph’s Health Care London, as well as the hospital foundations (London Health Sciences Foundation and St. Joseph’s Health Care Foundation).
Institutional Research Data Management Strategy
Table of Contents
3 Research Data and Importance of Research Data Management. 2
6.1 Awareness-Raising Activities. 4
6.3 Promote and Support RDM Practices. 5
6.4 Access to RDM Tools, Resources, and Infrastructure. 5
6.4.1 Information Technology (IT) Infrastructure. 5
9 Indigenous Data Considerations. 8
10 Other Relevant Strategies and Policies. 9
10.1 London Health Sciences Centre Corporate Policies and Procedures. 9
10.2 St. Joseph’s Health Care London Policies and Procedures. 10
10.3 External Strategies/Policies. 10
11 Acronyms & Abbreviations. 12
Introduction
The federal research funding agencies (Tri-Agency: Canadian Institutes of Health Research (CIHR), Natural Sciences and Engineering Research Council of Canada (NSERC), Social Sciences and Humanities Research Council (SSHRC)) released a Research Data Management Policy in March 2021. They have set a deadline for March 1, 2023, for each postsecondary institution and research hospital eligible to administer CIHR, NSERC, or SSHRC funds to develop an institutional strategy for Research Data Management (RDM), and notify the agencies when it has been completed. Additionally, for specific funding opportunities, the agencies require data management plans (DMPs) to be submitted to the appropriate agency at the time of application. Furthermore, grant recipients are required to deposit into a digital repository all digital research data, metadata, and code that directly support the research conclusions in journal publications and pre-prints that arise from agency-supported research.
Lawson Research Institute is the research arm of St. Joseph’s Health Care London. Lawson recognizes the impact of funding on research, the RDM requirements and obligations implemented by funding agencies, and the importance of research data management. Lawson is fully engaged in developing and implementing the Institutional RDM Strategy.
The Tri-agency is committed to funding research that is conducted to the highest professional and disciplinary standards, is performed ethically, makes effective use of public funds, is verifiable and replicable, and that makes results as accessible as possible. The agencies support the FAIR (Findable, Accessible, Interoperable, and Reusable) guiding principles and accordingly advocate an increased ability for research data to be archived, found, and responsibly reused to fuel discoveries and innovation across multiple disciplines and geographical borders. Research data management is, therefore, a necessary component of achieving research excellence.
Research data is the data used as evidence to support and validate research findings or results and used as input for analysis. Research data is derived from source data. This can include information extracted from original sources such as clinical systems, experiments, simulations, etc. It is to be noted that any data containing identifiable personal information must remain private and confidential.
Research Data Management is the organization and maintenance of research data throughout the entire research project lifecycle. This includes setting up protocols before initiating data collection, and then collecting, tracking, and creating backups of the data during study execution, and eventually, data sharing, archiving, and publishing upon project completion. This is not a new concept. In fact, Lawson researchers have been employing these processes and procedures and performing RDM in varying capacities. However, with the new policy requirements, obligations for regulatory compliance, concerns for privacy and security, initiatives for data sharing and reproducibility, a push for the FAIR principles, the open science movement, and a need to elevate the availability of Canadian data on the world stage, it is imperative for Lawson to implement and support RDM best practices and procedures.
The Lawson institutional RDM strategy is a concise and directive document that outlines how Lawson will increase its capacity to support and foster a culture of effective research data management. This institutional strategy is a collaboration between internal and external key stakeholders. It will support Lawson researchers in managing their data throughout the research lifecycle using appropriate data stewardship and data management practices.
This strategy applies to all research data generated and collected by Lawson researchers, research trainees, and research staff, whether the research was funded by the Tri-agency or other funders, or self-funded.
This strategy does not propose the creation of new or amendment of existing hospital policies.
The RDM Advisory Committee supports establishing and implementing the overall institutional strategy. Drafting of the institutional RDM strategy is being led by the RDM Project Team, comprising Research Informatics, Grants Development, Quality Assurance, and Research Administration team members through Lawson and (jointly with London Health Sciences Centre Research Institute) through a shared Office of Research Services
The RDM Advisory Committee, comprising key institutional stakeholders, acts as a resource to the RDM Project Team on the planning, implementation, and ongoing evaluation of the strategy. These stakeholders include Lawson’s Chief Operating Officer, representatives from the hospital Privacy Offices, Information Technology Services, LHSC Data Governance, Research Ethics Boards, Western Libraries, Lawson Approvals, Grants Development, Quality Assurance, Research Directors, research teams, and research trainees. The RDM Project Team consults with other stakeholders and community partners as needed to support the RDM rights of all stakeholders involved in research.
The Advisory Committee further understands that as the research landscape advances, the RDM requirements and obligations implemented by Tri-agency and other funders may change; as RDM progress is made as outlined in this strategy, the resources and priorities will also change, necessitating re-evaluation of RDM maturity and revision of this RDM strategy. Hence, the strategy will be considered a living document that will be reviewed on an annual basis by the Advisory Committee.
Lawson aims to meet RDM requirements and implement RDM best practices and processes to fully support its researchers and research communities. Through the implementation of the institutional RDM strategy, Lawson will provide sustainable support and solutions by documenting existing support and processes, formalizing responsibilities, and expressing and promoting RDM best practices. Lawson aims to support researchers in establishing and implementing data management practices consistent with ethical, legal, and commercial obligations.
6.1 Awareness-Raising Activities
The Lawson research community was engaged through various means to raise awareness about the Tri-Agency RDM policy requirements and RDM.
Data champions were recruited from different departments to help promote the value of RDM and engage with various communities.
The research community was invited to participate in an anonymous survey to understand the current state of Lawson in developing and allocating human, organizational, infrastructure, and financial resources for Research Data Management within the Lawson research community.
The RDM Maturity Assessment, based on Maturity Assessment Model in Canada (MAMIC), was conducted to take stock of the current services and identify areas for future growth and development. It helped to capture the perceptions of the current RDM service offerings.
Lawson also established an internal RDM website to provide information about research data management, Tri-Agency RDM Policy requirements, data management plans, institutional RDM strategy, frequently asked questions, and resources. This website provides up-to-date information and keeps the research community informed.
The RDM Project team hosted webinars on research data management, data management plan templates, and DMP Assistant.
The objective is to assist the broader research community in understanding the institution’s current and planned RDM capacity, challenges, and needs. Therefore, to facilitate an ongoing dialogue and collaboration on the advancement of RDM on a national level, Lawson has created web pages dedicated to RDM on an external-facing website.
Lawson has research data management expertise and skills within different departments. However, a centralized and integrated approach to support RDM is required. Infrastructure support for large data sets can also generate some human resources issues. New research data management approaches also require the upkeep of skills, techniques, processes, and solutions. Accordingly, appropriate knowledge and skill development will be needed for the team to support RDM.
6.3 Promote and Support RDM Practices
Lawson will continue to support researchers and their staff by encouraging a data management culture and an environment that promotes and facilitates research data management. Lawson’s Research Informatics and Grants Development teams will provide Data Management Plans and data deposit consultation services.
6.4 Access to RDM Tools, Resources, and Infrastructure
6.4.1 Information Technology (IT) Infrastructure
Lawson’s IT infrastructure is managed and supported by the hospitals’ Information Technology Services (ITS) department.
ITS provides access to OneDrive, Teams, SharePoint, Webex, Office 365, MS Project, and many more solutions and platforms available for research purposes.
All systems available to Lawson researchers through ITS are backed up on a nightly and monthly basis. Backups on tapes are also stored at an off-site location for disaster recovery.
Lawson-supported servers are hosted at the secure ITS data centre. These servers are configured and secured as per hospital guidelines.
Various safeguards have been implemented and documented to prevent, detect, and mitigate the effects of computer viruses, worms, or other potentially harmful software code on study data and software.
The virtual servers hosted at the hospitals’ data centre employ vMotion. It allows IT to move running virtual machines from one physical server to another without impacting end-users. vMotion keeps the IT environment up and running, providing unprecedented flexibility and availability. It also decreases downtime and improves reliability by supporting business continuity and disaster recovery procedures.
Lawson researchers have access to many support services through different departments.
The RDM Project team hosts workshops related to RDM and DMPs.
The Research Informatics team hosts workshops to build capacity in researchers to accelerate the build of high-quality data capture projects.
Quality Assurance and Education Program hosts ‘Lunch ‘n Learn’ sessions related to policies and regulations, and educates researchers and staff on compliance requirements and best practices.
ITS has partnered with Microsoft and peer Ontario hospitals to develop Live Virtual Training sessions to allow learning at an individual pace.
Several tools are available to Lawson researchers to support their RDM requirements.
REDCap is a Research Electronic Data Capture web-based tool for creating and managing online database applications and surveys. Hosted at the hospitals’ data centre, Lawson Research Informatics administrates this secure platform to meet the diverse research needs of the Lawson community.
Lawson supports its researchers by providing robust infrastructure to support their research activities on a safe, secure IT platform to ensure patient confidentiality for clinical activities; and by providing hosting for customized web applications for research studies. Lawson manages several research applications on Windows and Linux servers, securely hosted at the London hospitals’ data centre.
File Safe and M365 are the recommended tools available to Lawson researchers to securely transfer files, large and small, including confidential or patient-identifiable information, instead of using email attachments.
DMP Assistant is a national, online, bilingual data management planning tool to assist researchers in preparing data management plans (DMPs). Lawson researchers and their team members can create an account on this platform. They can select ‘Lawson Health Research Institute’ as their organization to develop their discipline or study-specific Data Management Plan through a series of critical data management questions supported by best-practice guidance and examples.
Lawson researchers also have access to the Lawson DMP Template that guides them through all the elements of a data management plan and provides example answers to several questions.
Canadian Repository Options
Borealis, a publicly accessible, secure Canadian data repository system managed by Western Libraries, allows for data to be released and shared openly or privately with precision at the file level using Dataverse software. This is available to Lawson researchers who are faculty members at Western University.
Lawson researchers can utilize the national Federated Research Data Repository (FRDR) platform to deposit data or to search for and download data across Canadian repositories. This platform can efficiently ingest datasets of any size, and preservation processing is done automatically. Research data can be ingested, curated, preserved, discovered, cited, and shared from this single platform.
International Repository Options
The Directory of Open Access Repositories (OpenDOAR) provides a quality-assured list of open-access repositories worldwide. OpenDOAR staff harvest and assign metadata to allow categorization and analysis to assist the wider use and exploitation of repositories. OpenDOAR is based at the University of Nottingham.
Re3data.org is a global registry of research data repositories that covers research data repositories from different academic disciplines. It includes repositories for the permanent storage and access of data sets to researchers, funding bodies, publishers, and scholarly institutions. Re3data.org promotes a culture of sharing, increased access, and better visibility of research data.
Several key stakeholders were identified internally and externally from the organization. The RDM Advisory Committee was formed in March 2022 to include relevant stakeholders who are directly impacted by the implementation of the Institutional Strategy. This committee included stakeholders from Executive Administration, Research Informatics, Grants Development, and representatives from London Health Sciences Centre and St. Joseph’s Privacy Offices, Information Technology Services, LHSC Data Governance, Western University’s Health Sciences Research Ethics Board, Western Libraries, Lawson Approvals, Quality Assurance, Research Directors, Research teams, and a Postdoctoral Fellow. The Advisory Committee meets monthly to help raise awareness, assess institutional readiness, and serve as a communication medium. Appropriate delivery mechanisms for outreach were implemented to engage the Lawson research community. The input and feedback from the research community were solicited through surveys, webinars, online RDM sites, email, ad hoc meetings, etc.
Perceiving the significance of collaboration with external stakeholders and community partners, the RDM Project Team has been reaching out to Indigenous Cancer Care Unit Clinical Institutes & Quality Programs and the Office of Inclusion & Social Accountability (Indigenous Health) at LHSC, and the Knowledge Exchange, Impact & EDI-D in Research office and Indigenous Health Lab at Western University for finding a common intersection of work and continued consultation and consideration concerning RDM training and processes.
Lawson Research Institute supports researchers in adopting and complying with ethical, legal, and commercial obligations through various means. Research oversight and compliance are overseen by Western University’s Health Sciences Research Ethics Board (HSREB), Clinical Trials Ontario (CTO), and Ontario Cancer Research Ethics Board (OCREB). They also oversee the ethical conduct of research studies involving human participants. Additionally, the Tri-Agency’s Tri-Council Policy Statement: Ethical Conduct Involving Humans – TCPS2 (2018) provides guidance to researchers conducting research involving human participants.
Lawson’s Quality Assurance and Education Program (QAEP), which is a part of Lawson’s Quality Management System (QMS), facilitates research compliance across the organization through Standard Operating Procedures, Guidance Documents, Lunch and Learns, Clinical Research Training, Quality Assurance Reviews as well as providing research support to Investigators, research teams and other stakeholders on the regulations, policies and best practices governing clinical research.
Research compliance with legal and commercial obligations falls under the Lawson Research Approval Systems (Contracts) and WORLDiscoveries, a joint business development arm for Lawson, Western University, and Robarts Research Institute. Lawson’s Contracts team is responsible for drafting, reviewing, negotiating, and coordinating all contracts for research under Lawson’s auspices.
Lawson intends to support researchers involved with Indigenous research and ensure that Tri-Agency RDM policy requirements are addressed. We recognize that there are many Indigenous communities, peoples, cultures, languages, and protocols and therefore no singular approach can be applied. We also acknowledge the validity of Indigenous epistemologies and ontologies.
Lawson recognizes, supports, and respects Indigenous data sovereignty and their right to own, control, access, possess, and protect the information collected from these communities, based on free, prior, and informed consent. We are committed to respect and adhere to nation and community specific protocols by following research data management principles developed and approved by these communities, collectives and organizations such as the First Nations Information Governance Centre’s OCAP (Ownership, Control, Access, Possession) principles, the Inuit Tapiriit Kanatami National Inuit Strategy on Research, and Global Indigenous Data Alliance’s CARE principles. These govern data collection, ownership, protection, use and sharing to encourage inclusive development and innovation, and equitable outcomes. Lawson will ensure that the DMPs are co-developed with these communities, collectives, and organizations, in line with RDM principles and DMP formats that they accept. We acknowledge that they have the right to repatriate the data and this could result in exceptions to the data deposit requirement.
Lawson researchers are also guided through TCPS 2 (2018) – Chapter 9: Research Involving the First Nations, Inuit and Métis Peoples of Canada to ensure that research involving Indigenous peoples is postulated on respectful relationships that encourage collaboration and engagement between researchers and participants. It is a policy that serves as a framework for the ethical conduct of research involving Indigenous peoples in Canada.
It is our institutional responsibility to build capacity for doing this work in an effective way. Lawson is working with the Indigenous Cancer Care Unit Clinical Institutes & Quality Programs and the Office of Inclusion & Social Accountability (Indigenous Health) at LHSC. Lawson is also collaborating with the Office of Equity, Diversity, and Inclusion (EDI) and the Indigenous Health Lab at Western University.
Lawson aims to strengthen Indigenous research capacity by facilitating and promoting equitable access and support for Indigenous students and researchers. Lawson Scientists usually have Western faculty appointments and/or have employment as clinicians with St. Joseph’s. Lawson supports Indigenous researchers with these affiliations and encourages non-Indigenous researchers to co-develop new models for Indigenous research and research training with Indigenous communities. This may include co-developing research questions, agendas, respectful relations, and impactful solutions built on trust, respect, and mutual interests. Indigenous researchers and leadership will help non-Indigenous researchers understand Indigenous perspectives, needs, concerns and aspirations for Indigenous research. Indigenous researchers can help foster positive collaboration with Indigenous partners, collect organic responses from Indigenous participants and provide an Indigenous research lens on the collected information and analyzed data.
These efforts will help us create research data management guidelines for our researchers involved in Indigenous research, assuring the best practices reflect the Four R’s - Respect, Relevance, Reciprocity, and Responsibility.
Lawson Research Institute is governed by St. Joseph’s policies, processes, and procedures that are relevant to various aspects of RDM.
The Lawson RDM Strategy is also intended to align with external requirements and guidance, including provincial, federal, and international laws.
The pertinent policies and documents that were reviewed are listed below.
10.1 London Health Sciences Centre Corporate Policies and Procedures
Acceptable Use of Information Technology Resources |
Breach of Privacy |
Privacy |
Confidentiality |
Electronic Mail (Email) Use |
Acceptable Use of Information Technology Resources |
Records Retention and Disposition |
Remote Access to Computer Network Resources |
Security of Confidential Information and Information Technology Systems |
Use of Cellular Phones and Other Wireless Devices |
Use of Personal Health Information for Research, Education, and Quality Improvement |
Invention - Lawson |
Lawson Approval for Clinical Research |
10.2 St. Joseph’s Health Care London Policies and Procedures
Acceptable Use of Information Technology Resources |
Access and Disclosure of Personal Health Information |
Breach of Patient Privacy |
Clinical Trials Involving Investigational Drugs |
Confidentiality |
Disclosure of Patient Information, Samples, and/or Belongings to Law Enforcement Agents |
Electronic Mail (Email) Use |
Freedom of Information and Protection of Privacy Act (FIPPA) |
Health Record Management |
Interpretation and Translation Services |
Patient Requests to Restrict the Use and Disclosure of Personal Health Information |
Privacy |
Records Retention and Destruction |
Remote Access to Computer Network Resources |
Security of Confidential Information and Information Technology Systems |
Use of Personal Health Information for Research, Education, and Quality Assurance |
10.3 External Strategies/Policies
Acronym/Term | Definition |
CARE | Collective Benefit, Authority to Control, Responsibility, and Ethics |
CIHR | Canadian Institutes of Health Research |
DMP | Data Management Plan |
EDI | Equity, Diversity, and Inclusion |
FAIR | Findable, Accessible, Interoperable, and Reusable |
HSREB | Health Sciences Research Ethics Board |
ITS | Information Technology Services |
Lawson | Lawson Research Institute of St. Joseph's Health Care London |
LHSC and LHSCRI | London Health Sciences Centre and its research arm, London Health Sciences Centre Research Institute |
MAMIC | Maturity Assessment Model in Canada |
NSERC | Natural Sciences and Engineering Research Council of Canada |
OCAP | Ownership, Control, Access, and Possession |
OCREB | Ontario Cancer Research Ethics Board |
QAEP | Quality Assurance and Education Program |
QMS | Quality Management System |
RDM | Research Data Management |
SSHRC | Social Sciences and Humanities Research Council |
St. Joseph’s | St. Joseph’s Health Care London |
Tri-Agency | CIHR, NSERC, SSHRC |
WORLDiscoveries | WORLDiscoveries is the commercialization arm of Western University, Robarts, and Lawson and represents our commitment to protecting and transferring technologies developed by our partners to market. |
The definitions below are as per the Tri-Agency Research Data Management Policy, Frequently Asked Questions, and Social Sciences and Humanities Research Council Definition of Terms, 2021, Government of Canada.
Data Deposit
“Data deposit” refers to when the research data collected as part of a research project is transferred to a research data repository. The repository should have easily accessible policies describing deposit and user licenses, access control, preservation procedures, storage and backup practices, and sustainability and succession plans. The deposit of research data into appropriate repositories supports ongoing data-retention and, where appropriate, access to the data.
Data Management Plan
A data management plan (DMP) is a living document, typically associated with an individual research project or program that consists of the practices, processes, and strategies that pertain to a set of specified topics related to data management and curation. DMPs should be modified throughout a research project to reflect changes in project design, methods, or other considerations.
Indigenous Research
Research in any field or discipline that is conducted by, grounded in or engaged with First Nations, Inuit, Métis or other Indigenous nations, communities, societies or individuals, and their wisdom, cultures, experiences or knowledge systems, as expressed in their dynamic forms, past and present. Indigenous research can embrace the intellectual, physical, emotional and/or spiritual dimensions of knowledge in creative and interconnected relationships with people, places and the natural environment.
Metadata
“Metadata” are data about data—data that define and describe the characteristics of other data. Accurate and relevant metadata are essential for making research data findable. A principle to help determine what information should be included in metadata is the open archival information system model criterion that the information be “independently understandable.”
Research Data
Research data are data that are used as primary sources to support technical or scientific enquiry, research, scholarship, or creative practice, and that are used as evidence in the research process and/or are commonly accepted in the research community as necessary to validate research findings and results. Research data may be experimental data, observational data, operational data, third party data, public sector data, monitoring data, processed data, or repurposed data. What is considered relevant research data is often highly contextual, and determining what counts as such should be guided by disciplinary norms.
Research Data Management
Research data management (RDM) refers to the processes applied through the lifecycle of a research project to guide the collection, documentation, storage, sharing, and preservation of research data.
The Lawson Research Data Management Strategy is a living document that will be reviewed and shared on an annual basis. It will be revised and updated as the institutional and researchers’ research data management requirements, practices, and understanding advance.
In the coming years, Lawson will:
evaluate the changes in policy requirements,
assess evolving institutional RDM training needs
gauge availability of the supporting resources
ensure compliance with the RDM policies and requirements by funders, publishers, and legislative bodies
evaluate technological infrastructure and meet expanding storage requirements – this may include assessment of access on platforms like Borealis for Lawson researchers who are not Western faculty members
facilitate capacity-building events for RDM and DMP development
provide training and educational services and support
examine DMP evaluation criteria and guidelines
consider requirements for data deposit certifications
adjudge implications on the involved stakeholders
consult with community stakeholders to confirm Indigenous data sovereignty considerations
It has been clear that implementation of the institutional RDM strategy requires collaboration on a broader scale. To that end, the RDM Project Team will build additional meaningful connections and identify subject matter experts for future RDM projects. Furthermore, the RDM project team is also proposing to establish a Community of Practice to manage RDM requirements in a sustainable method. This Community of Practice may comprise key stakeholders and experts from groups such as Ethics, Privacy, QA, Indigenous wellness group, Office of Inclusion and Social Accountability, research investigators, research coordinators, and research personnel.