Search
Search
Research Data Management
Research data management (RDM) is the organization and maintenance of research data throughout the entire research project life cycle. This includes setting up protocols before initiating data collection, and then collecting, tracking, and creating backups of the data during study execution. It also includes data sharing, archiving and publishing upon project completion.
RDM is not a new concept. Lawson Research Institute (Lawson) of St. Joseph’s Health Care London researchers already employ these processes and procedures, and perform RDM in varying capacities.
Why is it important?
RDM is an essential part of research excellence. Research must be conducted to the highest professional standard by ensuring that it is performed ethically, makes good use of public funds, experiments and studies are replicable, and research results are as accessible as possible. In addition, some journals require certain types of data to be shared or stored in specific repositories as a condition of publication. Concerns around reproducibility of research results have led to increased interest in data sharing so research results can be replicated and confirmed.
There is also a need to elevate the availability of Canadian data on the world stage. This means we need more Canadian datasets to be cited and used in research outputs and acknowledged appropriately. This would also increase the ability for research data to be archived, found, and responsibly reused, to fuel new discoveries and innovation across multiple disciplines and geographical borders.
In short, strong RDM practice is a sign of research excellence and Lawson is committed to the highest quality of research integrity and excellence.
Institutional RDM Strategy
An institutional RDM strategy is a concise and directive document that outlines how an institution will increase its capacity for effective RDM.
The purpose of creating and establishing an institutional RDM strategy is to foster a culture of sustainable and collaborative data stewardship and develop the capacity to support researchers in adopting responsible RDM practices, following FAIR (Findable, Accessible, Interoperable, and Reusable) guiding principles.
Lawson recognizes that efficient research data management is an essential element of research excellence. A Lawson Institutional Research Data Management Strategy has been developed in accordance with the Tri-Agency Research Data Management Policy (Government of Canada, 2021).
For additional information please contact: @email.
Research encourages re-evaluation of special nerve treatment for chronic pain
LONDON, ON – Hospital researchers from Lawson Health Research Institute have published a recent study that assessed the use of a specialized treatment for chronic pain and its impact on health care use and opioid prescribing.
Paravertebral blocks (PVBs) belong to a broader group of procedures called “nerve blocks.” A recent Toronto Star report noted that OHIP has been billed $420 million for nerve block procedures since 2011. PVBs involve injecting medication around the nerves where they exit the bones of the spine, at different locations depending on the patient and the chronic pain they are experiencing.
The regular use of these procedures has been questioned by health care providers due to the high cost and limited evidence of their benefit in reducing chronic pain. While the effectiveness of PVBs has been examined in trauma, cancer pain and regional anesthesia during surgery, they have not been evaluated for use in chronic pain despite widespread use in Ontario.
It is estimated that one in five Canadians live with chronic pain. Pain that persists can affect all aspects of someone’s life and health, particularly when it is not being managed.
This new study from London researchers found that 66,310 patients had a PVB between July 2013 and March 2018, and 47,723 patients were included in the study. In the year after a patient’s first PVB, there was a significant increase in the number of physician visits. Additional PVBs were frequently performed after the first treatment, with over 26 per cent of patients receiving a PVB ten or more times in one year, with almost eight per cent of patients receiving 30 or more. No overall change was found in opioid dosage in the year after PVB was initiated compared to the year before.
“Frequent use of PVB is common. Initiating treatment with PVCs is associated with marked increases in health care utilization, which includes physician visits and other injection procedures,” explains Dr. Eldon Loh, Lawson Associate Scientist and Physiatrist at St. Joseph’s Health Care London.
This research provides a broad perspective on the use of PVBs in Ontario, and on the use of nerve blocking treatments in general. There has been a concern for several years about the over use of these procedures; however, this is the first study to systematically document the impact on health care utilization and opioid use.
"We hope that from this study, the appropriate use of PVBs and other pain interventions will be re-evaluated at a provincial level to ensure the use of health resources is being properly managed and we achieve the best outcome for patients,” Dr. Loh adds.
The study, “A Retrospective Cohort Study of Healthcare Utilization Associated with Paravertebral Blocks for Chronic Pain Management in Ontario,” is published in the Canadian Journal of Pain.
-30-
Lawson Health Research Institute is one of Canada’s top hospital-based research institutes, tackling the most pressing challenges in health care. As the research institute of London Health Sciences Centre and St. Joseph’s Health Care London, our innovation happens where care is delivered. Lawson research teams are at the leading-edge of science with the goal of improving health and the delivery of care for patients. Working in partnership with Western University, our researchers are encouraged to pursue their curiosity, collaborate often and share their discoveries widely. Research conducted through Lawson makes a difference in the lives of patients, families and communities around the world. To learn more, visit www.lawsonresearch.ca.
Senior Media Relations Consultant
Communications & Public Engagement
T: 519-685-8500 ext. 73502
Celine.zadorsky@lhsc.on.ca
Research encourages re-evaluation of special nerve treatment for chronic pain
Hospital researchers from Lawson Health Research Institute have published a recent study that assessed the use of a specialized treatment for chronic pain and its impact on health care use and opioid prescribing.
Paravertebral blocks (PVBs) belong to a broader group of procedures called “nerve blocks.” A recent Toronto Star report noted that OHIP has been billed $420 million for nerve block procedures since 2011. PVBs involve injecting medication around the nerves where they exit the bones of the spine, at different locations depending on the patient and the chronic pain they are experiencing.
The regular use of these procedures has been questioned by health care providers due to the high cost and limited evidence of their benefit in reducing chronic pain. While the effectiveness of PVBs has been examined in trauma, cancer pain and regional anesthesia during surgery, they have not been evaluated for use in chronic pain despite widespread use in Ontario.
It is estimated that one in five Canadians live with chronic pain. Pain that persists can affect all aspects of someone’s life and health, particularly when it is not being managed.
“Frequent use of PVB is common. Initiating treatment with PVCs is associated with marked increases in health care utilization, which includes physician visits and other injection procedures,” explains Dr. Eldon Loh, Lawson Associate Scientist and Physiatrist at St. Joseph’s Health Care London.
This research provides a broad perspective on the use of PVBs in Ontario, and on the use of nerve blocking treatments in general. There has been a concern for several years about the over use of these procedures; however, this is the first study to systematically document the impact on health care utilization and opioid use.
"We hope that from this study, the appropriate use of PVBs and other pain interventions will be re-evaluated at a provincial level to ensure the use of health resources is being properly managed and we achieve the best outcome for patients,” Dr. Loh adds.
Research shared and celebrated at 17th Annual Mental Health Research Half Day
From falls prevention to depression therapies, scientists at Lawson Health Research Institute are conducting important mental health studies. Held on Thursday, September 15, the 17th Annual Mental Health Research Half Day at the Parkwood Institute Mental Health Program was a chance to share and celebrate this research.
The Mental Health Research Half Day featured poster and oral presentations, as well as the 12th Annual Tony Cerenzia Research Lecture. Clinical, administrative and research staff attended to learn more about research happening at Parkwood Institute and the Southwest Centre for Forensic Mental Health Care, part of the St. Joseph’s Health Care London family.
“The Mental Health Research Half Day provides an opportunity for researchers at Parkwood Institute and the Southwest Centre for Forensic Mental Health Care to present their research findings to clinical and administrative staff,” said Dr. Richard O’Reilly, Director of Psychiatric Research at Parkwood Institute & Southwest Centre and a Scientist at Lawson. “It is important that all clinical staff, who may not be directly involved in research, know what studies are being conducted and their impact on patient care.”
The 12th Annual Tony Cerenzia Research Lecture was delivered by Dr. Nathan Herrmann, Associate Scientist, Evaluative Clinical Sciences, Hurvitz Brain Sciences Research Program, Sunnybrook Research Institute and Head of the Division of Geriatric Psychiatry at Sunnybrook Health Sciences Centre. Dr. Herrmann delivered an engaging lecture titled “Managing Neuropsychiatric Symptoms in Dementia: An Evidence-Based Approach”.
Attendees were engaged not only by this highly informative lecture but also by the poster and oral presentations which covered a broad range of research topics. Presenters were also enthusiastic about the day and the opportunities it provided.
“The Mental Health Research Half Day is a great event which provides networking opportunities here at Parkwood Institute. It allows staff from across St. Joseph’s to learn about different research happening across program areas,” said Erin Finley, an Occupational Therapist (OT) in Geriatric Psychology at Parkwood Institute.
Finley and her colleagues were one of seven poster presentations. Their research project, titled “Fall prevention initiative in geriatric psychiatry”, aimed to reduce the rate of falls with injury among patients with dementia in a behavioural health unit. Within an 18-bed unit, they were able to significantly reduce falls with injury with zero incidences in the last two months of their data collection period.
Researchers developing photoacoustic hand-held probe for tumour detection during breast conserving surgery
Researchers at Lawson Health Research Institute (Lawson) are developing a hand-held photoacoustic imaging probe to be used during breast conserving surgery to quickly and accurately verify if all cancerous tissue has been removed.
Surgeons currently do not have real-time technology to guide tumour removal during surgery.
Using current tools, there is a 20 per cent chance that cancerous cells will be left behind, risking recurrence and repeat surgery.
Breast cancer represents 25 per cent of all new cancer diagnoses in women and 13 per cent of all cancer related deaths in women. Treatment for breast cancer often requires either complete breast removal in severe cases, or surgical removal of the cancerous tumour in combination with other therapies. Removing only the tumour is called breast conserving surgery.
Image


Photoacoustic Screening
The new device is an extension of the photoacoustic screening (iPAS) technology developed in the laboratory of Dr. Jeffrey Carson, Principal Investigator and Lawson Scientist. The technique uses light and sound to capture 3D images of surgically removed breast tissue. Their studies show that iPAS can catch up to 75 per cent of missed tumour cells, decreasing the odds of failed surgery to five per cent.
Dr. Muriel Brackstone, Associate Scientist at Lawson, Head of the Breast Care Clinic at St. Joseph’s Hospital London, and Surgical Oncologist at London Health Sciences Centre, brings her clinical expertise to the project.
“With the first generation iPAS technology, we would remove the tumour, take it to the lab for imaging and wait to see if there was a rim of normal tissue around the removed tumour so we knew it was removed completely. The wait was anywhere from 20 minutes to an hour. During that time, the patient is under anesthesia, the surgical team is idle and precious OR time is being used,” explains Dr. Brackstone.
A hand-held tool that surgeons can use
Creation of a hand-held probe to be used in the operating room is the next step in the advancement of this new technology. Elina Rascevska, biomedical engineering student at Western University, recently joined the Lawson team to convert lab-based iPAS technology into a hand-held device.
“We have developed a prototype of the iPAS probe, and once we can verify the quality of the images it produces, we will give it to Dr. Brackstone to test in the OR,” says Rascevska.
The iPAS probe does not need a trained operator and would be used by the surgical team. Instead of imaging the removed tissue, it scans the surgical cavity in real time to give the team a faster and more accurate indication as to whether the cancerous tissue has been removed.
“If we can progress this technology to a point where physicians can use it as part of standard protocols, we will have reduced the amount of time each patient needs to spend in the OR, the amount of call-backs and repeat surgeries, and ultimately improve quality of life for patients with breast cancer,” adds Dr. Carson.

(From left): Dr. Jeffrey Carson, Elina Rascevska, Dr. Muriel Brackstone
Researchers testing triple intervention to combat dementia
Researchers at Lawson Health Research Institute are the first in the world conducting a clinical trial to test a triple intervention aimed at treating Mild Cognitive Impairment (MCI) and delaying the onset of dementia. The Mobility, Exercise and Cognition (MEC) team will be incorporating physical exercises, cognitive training and vitamin D supplementation to determine the best treatment for improving mobility and cognition.
“We have learned the brain processes involved in motor-control - for example how a person walks - and cognition - for example how that person solves a problem - share similar locations and networks in the brain,” explains Dr. Manuel Montero Odasso, Lawson Scientist and Geriatrician at St. Joseph’s Health Care London. “Problems with mobility are connected to lowering function in the mind, and so can be a good indicator of future progression into dementia.”
Dr. Montero Odasso is also an Associate Professor in the departments of Medicine and Epidemiology and Biostatistics at the Schulich School of Medicine & Dentistry, Western University.

Gait assessment looks at the way in which we move our whole body from one point to another, helping to analyze mobility and the brain processes involved.
MCI is an intermediate stage between the expected cognitive decline of normal aging and the more serious decline of dementia. It can involve problems with memory, language, thinking and judgment. While many older individuals experience decline in both mobility and cognition, each are assessed and treated separately with no specific recommendations available for physicians.
The SYNERGIC Trial will combine physical exercises, cognitive training and vitamin D to test how these interventions work together to improve cognition in older adults at risk for dementia. The trial is targeting cognitive decline at the earliest stage, individuals with MCI, where interventions are more likely to have an effect and can be monitored.

Dr. Manuel Montero Odasso, Lawson Scientist and lead for the SYNERGIC Trial.
Dr. Montero Odasso explains that both physical and cognitive exercises have shown promising effects for maintaining cognition, while vitamin D deficiency is associated with cognitive decline. A key feature of this trial is that participants will receive individualized and progressive training.
“By delaying declines in cognition, we can improve a person’s quality of life. This research will help to support a more comprehensive preventative treatment with clinical guidelines for physicians whose patients are at risk of developing dementia,” states Dr. Montero Odasso. “Even more, each one year delay of progression to dementia in older individuals at risk has the opportunity to save billions of dollars for the Canadian health care system.”
Individuals over 60 years old with mild cognitive impairment without dementia are eligible for this clinical trial. Those interested in participating are encouraged to contact Research Coordinator Alanna Black at 519.685.4292 ext. 42179.
Participants will be asked to complete a routine of exercises and cognitive training three times a week for six months, with one final assessment at 12 months. The main site for the study is Parkwood Institute with physical exercises taking place at the Labatt Health Sciences Building at Western University, in Dr. Kevin Shoemaker’s Laboratory for Brain and Heart Health.
This study has been funded by the Canadian Consortium on Neurodegenerative in Aging (CCNA) which represents Canada-wide research aimed at enhancing the quality of life and services for individuals diagnosed with a neurodegenerative disease. The MEC team in London, led by Dr. Montero Odasso, includes expert researchers in the field of mobility who aim to develop common assessments for the interaction of cognition and mobility for older people to aid as a diagnostic tool for detecting dementia.

Members of the study’s research team, from left to right: Korbin Blue, Research Assistant (Co-op Student); Yanina Sarquis-Adamson, Lab Research Assistant; Frederico Faria, Post-Doctoral Fellow; Dr. Montero Odasso, Director, Gait and Brain Lab; research participant; Alanna Black, Lab Research Coordinator; Stephanie Cullen, Research Assistant (Undergraduate Student); and, Navena Lingum, Research Assistant (Master Student).
Researchers using MRI scans to pinpoint moral injury effects in health care workers
Mental health concerns have been on the rise amongst health care workers during this ongoing pandemic. With long hours, fears of the unknown, and the pressure of keeping themselves and their families safe, some health care workers have suffered a moral injury.
Moral injury refers to an injury to an individual’s moral conscious, which can produce profound emotional guilt and shame. Recognizing this is a growing concern, a London research team from Lawson Health Research Institute and Western University’s Schulich School of Medicine & Dentistry is examining moral injury amongst health care workers by imaging the effects on the brain.
“We are trying to look closely at what happens in the brain when a person recalls a moral injury event,” says Dr. Ruth Lanius, Associate Scientist at Lawson and Professor at Schulich Medicine & Dentistry. “By understanding the changes happening in the brain, we may be better able to treat individuals suffering from moral injury.”

Dr. Ruth Lanius, Lawson Associate Scientist/Professor Schulich School of Medicine & Dentistry
During this ongoing pandemic, some health care workers have experienced emotionally difficult situations that resulted in moral injury. “Those suffering from moral injury have a cognitive or thinking component which may include repeated thoughts that they didn’t provide the best care for example, or that they let their family down due to their intense work schedule or need to self-isolated,” explains Dr. Lanius, who is also a psychiatrist at London Health Sciences Centre (LHSC).
“These thoughts are coupled with intense visceral distress, a gnawing sensation in the stomach or the feeling like one is being eaten up inside. I think once we help resolve the visceral distress, we will also see the negative thinking patterns settle down.”
The new study will involve around 60 health care workers. These research participants will undergo a functional MRI scan at St. Joseph's Health Care London at the beginning of the study and have the option to receive eight weeks of treatment. Then, another MRI scan will be done to see if and how the moral injury changes and possibly resolves within the brain. “This can be very validating for the health care workers since brain scans can make the invisible wound of moral injury visible,” adds Dr. Lanius.
The research team’s goal is to better understand what networks of the brain are activated with moral injury. Dr. Lanius hopes this would help establish more neuroscientifically guided treatments. “We have to help our health care workers heal from the tremendous hardships they often endure.”
Health care workers are still being recruited for this study. Interested participants can contact Research Coordinator Suzy Southwell 519-685-8500 ext. 35186 or @email.
Researching treatments for COVID-19
As we continue to live with a COVID-19 pandemic, patients will need good treatment options.
Hospital researchers in London, Ontario, through Lawson Health Research Institute, are testing treatment options for patients who have been hospitalized from a COVID-19 infection, often for severe symptoms.
During the peaks of the COVID-19 pandemic, there were concerns of a global drug shortage when it came to IV sedatives for patients needing ventilation. Through funding from the Government of Ontario’s COVID-19 Rapid Research Fund, a team of Ontario researchers studied whether inhaled sedatives could replace those that are delivered through IV.
“In addition to easing the burden on IV stocks, the inhaled sedatives have additional benefits,” says Dr. Marat Slessarev, a Critical Care Physician at LHSC. “They may reduce inflammation in the lungs and shorten the duration of sedation because they are eliminated from the body faster than IV sedatives.”
Dr. Slessarev, also a Scientist at Lawson, adds that using inhaled sedatives could also be safer for severe COVID-19 patients, who in many cases are on a ventilator for a long time. “When using IV sedatives, they can accumulate and metabolize with accumulation. This can leave the patient with the potential of developing kidney issues because it is hard to dispel them quickly.”

Dr. Marat Slessarev, Critical Care Physican at LHSC and Lawson Scientist
To date, around 800 patients across ten hospital sites have been enrolled in this trial, with the hopes of getting more hospital sites on board as the clinical trials continue.
A majority of people with severe COVID-19 infections in critical care end up developing sepsis, which is a potentially life-threatening condition that occurs when the body's response to an infection damages its own tissues.
“An inflammatory response is what the body uses to fight an infection, but sometimes it is more than necessary and it causes damage to the organs and the body,” explains Dr. Claudio Martin, a Physician in the Intensive Care Unit (ICU) at London Health Sciences Centre (LHSC). “This is why a patient with a severe COVID-19 infection caused by a virus can get sepsis.”
Dr. Martin, who is also an Associate Scientist at Lawson, began studying the use of a human protein called Annexin A5 as a potential treatment for COVID-19 patients with sepsis. “It can work in two ways. The annexin may coat injured cells and reduce the inflammatory response. Or, the injured cells and exposed proteins trigger the clotting mechanism in the body. Those with COVID-19 could get blood clots in the brain and the lungs, and using annexin may improve their outcomes.”

Dr. Claudio Martin, Critical Care Physician at LHSC and Lawson Scientist
Currently, a clinical trial using Annexin A5 on severe COVID-19 patients is underway at LHSC to look at the efficacy of using this human protein.
The COVID-19 virus is also known to cause respiratory failure, prompting Dr. Jim Lewis, Respirologist at St. Joseph’s Health Care London and Scientist at Lawson, to investigate the use of pulmonary surfactant as a potential treatment for these patients.
Bovine Lipid Extract Surfactant Suspension (BLES) is a pulmonary surfactant manufactured in London, Ontario. It’s currently used worldwide to help improve lung function in premature babies. Now, it is being studied with COVID-19 patients experiencing respiratory failure.

Dr. Jim Lewis, Repirologist at St. Joseph's Health Care London and Lawson Scientist
“We know that patients who have injury to their lung and require ventilation have inflammation in the airways of their lungs. Surfactant is a homogeneous layer of lipids and proteins that line the lungs to allow us to breath with minimal effort,” explains Dr. Lewis. “If we gave these patients surfactant as soon as possible after they were put on a mechanical ventilator, it may have some benefit in improving their outcome and getting them off the ventilator sooner.”
Dr. Lewis and his team conducted a clinical trial using pulmonary surfactant with ten critically ill COVID-19 patients and initial results have shown patient safety and efficacy.
Another team of hospital researchers turned to using modified dialysis machines to offer treatment options to those with severe COVID-19 symptoms. Nephrologist at LHSC, Dr. Chris McIntyre, was the first in the world to modify a dialysis device to treat a patient with COVID-19. The device gently removes a patient’s blood, modifies white blood cells, and returns them to fight hyperinflammation.
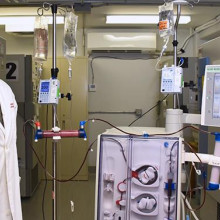
Dr. Chris McIntyre, Nephrologist at LHSC and Lawson Scientist
“It was quick. We went from the initial idea to the approvals, creating the device and appointing the first patient within 40 days,” says Dr. McIntyre who is also a Scientist at Lawson. “After the first 12 patients, we found that those treated with the device needed significantly less drugs to maintain their blood pressure. We were able to deliver the treatment for all the patients – none of the treatments failed and we had no safety issues.”
Dr. McIntyre is now looking into using this form of therapy for chronic dialysis patients to help modify organ injury.
Responding to the call for action during the COVID-19 pandemic
Quickly after the COVID-19 pandemic began to grip the world, Lawson Health Research Institute responded with action. Lawson is the research arm of London Health Sciences Centre (LHSC) and St. Joseph’s Health Care London. With hospitals focused on providing excellent patient care in the face of an unknown virus, hospital researchers in London, Ontario began critical COVID-19 studies.
“A lot was unknown during the first wave of the pandemic. Any research that wasn’t essential was put on hold. At the same time, we had people with different expertise coming together with different perspectives to see how we could better understand the SARS-CoV-2 virus and the COVID-19 infection,” explains Dr. David Hill, Lawson Scientific Director and VP, Research at LHSC and. St. Joseph's. “Our hospital researchers formulated ideas and very quickly came up with research proposals. These were fast tracked through our processes and within two months we approved over 50 new studies surrounding COVID-19.”

Dr. David Hill, Lawson Scientific Director and VP, Research at LHSC and. St. Joseph's
Some of these studies were clinical trials, which are research studies performed with people. Many patients from London hospitals get involved as patient participants and when COVID-19 hit, many agreed to be a part of this important research.
“We saw the pandemic happening across the world, and suddenly it was happening here in London. This has probably been the biggest challenge of my career,” says Carol Young-Ritchie, Executive Vice President at LHSC. “We had to look at many of our processes and how we were doing things, and adjust appropriately and nimbly.”
Young-Ritchie adds that as the hospital continued to admit a growing number of COVID-19 patients, a strong focus on research was needed. “It was absolutely critical and important for LHSC as a leader and academic centre to contribute to our collective knowledge. We needed to keep that research going and although it has been challenging, it has also taught us to be innovative.”

Carol Young-Ritchie, Executive Vice President at LHSC
The same focus was happening at St. Joseph’s Health Care London, with health care providers and researchers finding ways to improve care and outcomes for patients who had contracted the virus. “COVID-19 research through the hospital has been incredibly important,” says Karen Perkin, Vice President of Patient Care at St. Joseph’s. “We had researchers busy looking at the impacts of COVID-19. We had patients on ventilators and we were trying to understand that more. We also had research looking at the impacts for staff members looking after patients. All important, helpful knowledge as we move forward.”

Karen Perkin, Vice President of Patient Care at St. Joseph’s
Hospital research in London through Lawson is proudly affiliated with Western University. At Western’s Schulich School of Medicine & Dentistry, the new state-of-the-art Imaging Pathogens for Knowledge Translation (ImPaKT) Facility was the perfect environment to conduct COVID-19 research.
“ImPaKT is a special containment facility where research on viruses like SARS-CoV-2 can be done safely,” says Dr. David Litchfield, Vice Dean of Research and Innovation at Schulich Medicine & Dentistry. “It has become a focal point for dozens of studies involving research through Schulich Medicine & Dentistry, as well as partners from the hospitals and other academic institutions and industries.”

Dr. Litchfield adds that collaboration between hospital partners and scientists has been the key to successful COVID-19 research. “This collaboration has enabled advances leading to new diagnostic testing for COVID-19, as well as studies using MRI or related imaging tools to investigate long-term impacts of the infection on individuals.”
The rapid research response to COVID-19 couldn’t have happened without community and foundation financial support. “Funding support from our hospital Foundation is something we are so grateful for,” says Perkin, referring to St. Joseph’s Health Care Foundation. “They came right out at the beginning and asked how they could help, as did our donors.”
LHSC’s London Health Sciences Foundation and Children’s Health Foundation were also pivotal in research funding during the pandemic. “Funding is a crucial part to how we do hospital research and the Foundations have been important partners in making sure our research continued,” says Young-Ritchie.
As the pandemic continues, so does the research within our community. Hospital research has already improved diagnostics, treatments and patient outcomes related to COVID-19 and helped people all around the world.
“If you look at some of the achievements that have occurred in just a little more than a year, we have had a number of landmark publications on ways to diagnose COVID-19 compared to other respiratory disorders,” notes Dr. Hill. “We have had many rapid advances and it can take a crisis to bring out the best in people. Then things come together quickly, such as expertise, talent and money – and the job gets done.”