Search
Search
Monitoring the effects of COVID-19 quarantine measures on young adults with mood and anxiety disorders
LONDON, ON – Concerned that patients from the First Episode Mood and Anxiety Program (FEMAP) at London Health Sciences Centre (LHSC) would lose connection to important mental health services during the first wave of the pandemic, researchers at Lawson Health Research Institute (Lawson) tested the use of an electronic questionnaire to help monitor and assess the mental health impacts of isolating public health measures on young adults with mood and anxiety disorders.
Since the pandemic’s start, there have been concerns about the effects of quarantine measures on mental health. Young adults between the ages of 16 and 25 with mood and anxiety disorders are particularly vulnerable as they could experience high levels of depression, anxiety, traumatic stress and functional impairment from social isolation.
“It was unclear how these young adults would weather prolonged physical distancing, inactivity and reduced structure to their days. Some may be at an increased risk for depression while others may see symptoms improve due to fewer social expectations,” explains Dr. Elizabeth Osuch, Scientist at Lawson and Medical Director at FEMAP. “It is critical we understand how they respond to inform mental health care in response to the pandemic.”
The study followed 114 participants who completed regular surveys answering questions about their experiences during the pandemic, including information about their mood and anxiety symptoms, functioning and coping strategies. The team was immediately alerted if a patient’s survey responses were concerning, so they could reach out.
The research team analyzed changes in patient symptoms, functioning and coping strategies over the course of several months. Participants who were flagged for concerning scores were found to be younger, more likely to be on a waiting list for treatment, and more likely to have been laid off from work or have a higher degree of functional impairment.
“The questionnaire made it easy to stay connected with patients, and by monitoring their symptoms and functioning we were able to make sure that our resources, limited by the pandemic, could be directed to those who needed it the most,” says Dr. Osuch.
The researchers anticipated early on that those with mood and anxiety disorders would respond uniquely to the pandemic situation. Some would have more difficulty with the quarantine itself while others might ultimately find the return to normal more challenging. By understanding these different trajectories and how to track them, care can be optimized for future pandemic events or other public health emergencies.
The study, “Monitoring the effects of COVID‐19 in emerging adults with pre‐existing mood and anxiety disorders,” is published in Early Intervention in Psychiatry.
-30-
Lawson Health Research Institute is one of Canada’s top hospital-based research institutes, tackling the most pressing challenges in health care. As the research institute of London Health Sciences Centre and St. Joseph’s Health Care London, our innovation happens where care is delivered. Lawson research teams are at the leading-edge of science with the goal of improving health and the delivery of care for patients. Working in partnership with Western University, our researchers are encouraged to pursue their curiosity, collaborate often and share their discoveries widely. Research conducted through Lawson makes a difference in the lives of patients, families and communities around the world. To learn more, visit www.lawsonresearch.ca.
Senior Media Relations Consultant
Communications & Public Engagement
T: 519-685-8500 ext. 73502
Celine.zadorsky@lhsc.on.ca
Most instructions for inserting COVID-19 nasopharyngeal swabs don't go deep enough, research finds
LONDON, ON - There are wide discrepancies in the instructions for how deep the nasopharyngeal swabs used to test for COVID-19 are to be inserted up Canadian noses, new research from Western University and Lawson Health Research Institute has found.
As an otolaryngologist Dr. Leigh Sowerby is an expert in the anatomy of the head, neck and inside of the nose. Using that expertise, he and his colleagues examined the COVID-19 testing instructions provided by provincial and territorial authorities and found wide variations. They reported their findings in the Journal of Otolaryngology – Head & Neck Surgery.
“As a surgeon who works inside the nose all the time, I was surprised to find that most of the instructions in Canada aren’t effective to reach the nasopharynx; they just don’t go deep enough into the nasal cavity,” said Sowerby, an associate professor at Western’s Schulich School of Medicine & Dentistry and Scientist at Lawson Health Research Institute.
To perform a nasopharyngeal test, the swab must be inserted far enough into the nasal cavity to reach the nasopharynx, the upper part of the pharynx at the top of the throat behind the nose. Samples from the nasopharynx have been shown to be the most sensitive for COVID-19 testing, and are considered the gold standard.
However, less than a quarter of provincial and territorial public health instructions tell practitioners to insert the swab deep enough to reach the nasopharynx, Sowerby said.
The research found that six provinces and territories, including the Northwest Territories, Nunavut, Ontario, Saskatchewan, Prince Edward Island and Alberta, recommended that the swab be inserted to a depth of four centimetres, or half the distance from nostril to ear. This depth only reaches the mid-nasal cavity, not the nasopharynx, he said.
British Columbia and Manitoba recommended a seven-centimetre depth of insertion, which is still not enough -- only reaching the posterior nasal cavity but not the nasopharynx.
In Nova Scotia and Newfoundland, the recommended depth of insertion was two-thirds of the distance from nostril to ear, which would effectively reach the nasopharynx, as would following the instructions in New Brunswick and Yukon to insert the swab from nostril to external ear canal.
“If we are doing what we are calling a nasopharyngeal swab, the technique for that should be standardized; there is no reason why there should be so much variability,” Sowerby said. “The take-home message is that if we want the most accurate test results, there is room for improvement in the test instructions. Otolaryngologists have a role to play, as we can provide a great service by actively engaging with our local and regional health authorities to train on proper technique and anatomical knowledge.”
-30-
MEDIA
Video: Nasopharyngeal swab depth of insertion

Dr. Leigh Sowerby, Associate Professor at Western’s Schulich School of Medicine & Dentistry and Scientist at Lawson Health Research Institute.
Click for larger image.
Lawson Health Research Institute is one of Canada’s top hospital-based research institutes, tackling the most pressing challenges in health care. As the research institute of London Health Sciences Centre and St. Joseph’s Health Care London, our innovation happens where care is delivered. Lawson research teams are at the leading-edge of science with the goal of improving health and the delivery of care for patients. Working in partnership with Western University, our researchers are encouraged to pursue their curiosity, collaborate often and share their discoveries widely. Research conducted through Lawson makes a difference in the lives of patients, families and communities around the world. To learn more, visit www.lawsonresearch.ca.
Western delivers an academic experience second to none. Since 1878, The Western Experience has combined academic excellence with life-long opportunities for intellectual, social and cultural growth in order to better serve our communities. Our research excellence expands knowledge and drives discovery with real-world application. Western attracts individuals with a broad worldview, seeking to study, influence and lead in the international community.
The Schulich School of Medicine & Dentistry at Western University is one of Canada’s preeminent medical and dental schools. Established in 1881, it was one of the founding schools of Western University and is known for being the birthplace of family medicine in Canada. For more than 130 years, the School has demonstrated a commitment to academic excellence and a passion for scientific discovery.
Senior Media Relations Consultant
Communications & Public Engagement
T: 519-685-8500 ext. 73502
Celine.zadorsky@lhsc.on.ca
Most instructions for inserting COVID-19 nasopharyngeal swabs don't go deep enough, research finds
There are wide discrepancies in the instructions for how deep the nasopharyngeal swabs used to test for COVID-19 are to be inserted up Canadian noses, new research from Western University and Lawson Health Research Institute has found.
As an otolaryngologist Dr. Leigh Sowerby is an expert in the anatomy of the head, neck and inside of the nose. Using that expertise, he and his colleagues examined the COVID-19 testing instructions provided by provincial and territorial authorities and found wide variations. They reported their findings in the Journal of Otolaryngology – Head & Neck Surgery.
“As a surgeon who works inside the nose all the time, I was surprised to find that most of the instructions in Canada aren’t effective to reach the nasopharynx; they just don’t go deep enough into the nasal cavity,” said Sowerby, an associate professor at Western’s Schulich School of Medicine & Dentistry and Scientist at Lawson Health Research Institute.
To perform a nasopharyngeal test, the swab must be inserted far enough into the nasal cavity to reach the nasopharynx, the upper part of the pharynx at the top of the throat behind the nose. Samples from the nasopharynx have been shown to be the most sensitive for COVID-19 testing, and are considered the gold standard.
However, less than a quarter of provincial and territorial public health instructions tell practitioners to insert the swab deep enough to reach the nasopharynx, Sowerby said.
The research found that six provinces and territories, including the Northwest Territories, Nunavut, Ontario, Saskatchewan, Prince Edward Island and Alberta, recommended that the swab be inserted to a depth of four centimetres, or half the distance from nostril to ear. This depth only reaches the mid-nasal cavity, not the nasopharynx, he said.
British Columbia and Manitoba recommended a seven-centimetre depth of insertion, which is still not enough -- only reaching the posterior nasal cavity but not the nasopharynx.
In Nova Scotia and Newfoundland, the recommended depth of insertion was two-thirds of the distance from nostril to ear, which would effectively reach the nasopharynx, as would following the instructions in New Brunswick and Yukon to insert the swab from nostril to external ear canal.

National research collaboration leads to Health Canada approval of life-saving radioisotope production
A Canadian consortium, which includes Lawson Health Research Institute (Lawson), TRIUMF, BC Cancer and Centre for Probe Development and Commercialization, is the first in the world to receive regulatory approval to produce the world’s most commonly used medical isotope, technetium-99m (Tc-99m), using small particle accelerators known as cyclotrons.
Tc-99m is used in tens of millions of nuclear medicine procedures globally each year. These include cancer scans, cardiac tests, as well as several other diagnostic procedures. As the world moves away from uranium-based nuclear reactors, there has been growing concern in the medical community of a global shortage of these life-saving compounds. This development helps secure a domestic supply of Tc-99m for Canadian patients.
For over a decade, Dr. Michael Kovacs, Director, Lawson Cyclotron & PET Radiochemistry Facility, and Steven Foster, Business Manager, Lawson Imaging, have been working on research that has contributed significantly to this major development. They have demonstrated the successful production of Tc-99m on a standard hospital-based cyclotron at Lawson, confirming that this technology can be used by almost half of the world’s already installed cyclotrons. Clinical trials were conducted across Canada and locally at St. Joseph’s Health Care London.
“In 2011, we received federal funding to see if we could develop a technology to produce Tc-99m in hospital cyclotrons,” explains Dr. Kovacs. “Canada’s Chalk River nuclear reactor was one of the world’s largest suppliers, and it was set to close in 2016. Cyclotron facilities offer a greener, safer, more sustainable approach for producing critical medical isotopes. Our goal was to find an alternative to the traditional means of producing this isotope, and we have been successful.”
Nuclear medicine is a functional imaging technique, meaning that it images biological function. Medical isotopes are converted to radiopharmaceuticals which get injected into the patient during a procedure. According to the specific biological properties of the isotope, they move throughout the body, rendering a 3D map of where the isotope has gone. This gives researchers and medical professionals valuable information of how various physiological processes are performing.
“Canada is a global leader in nuclear imaging technology. With the help of our collaborators across the country, we have home-grown technology to produce commercial quantities of Tc-99m on common cyclotrons,” adds Mr. Foster. “This technology has been patented and licensed to ARTMS Inc., a spin-off from the consortium, and is now being commercialized and sold throughout the world.”
The process was approved by Health Canada in November, 2020, and is expected to be deployed in British Columbia by 2022.
New biomarker speeds identification of lung disease
LONDON, ON- A new diagnostic method could help identify one of the deadliest types of interstitial lung disease (ILD) sooner, allowing for faster treatment and improved patient outcomes.
Idiopathic pulmonary fibrosis (IPF) is one of the most serious and common types of ILD, occurring most often in patients 60 and older with an average survival time of three to five years. At any given time roughly 300 patients are being treated for IPF in London, Ontario. Globally, it is the number one reason for lung transplants.
A new study published in Respiratory Research has found that testing for a protein called cyclin-dependent kinase inhibitor protein (p16) in biopsy tissue may help more accurately identify IPF.
Dr. Marco Mura, Associate Scientist at Lawson and Respirologist at London Health Sciences Centre (LHSC), together with Dr. Matthew J. Cecchini, Pathologist at LHSC, led the study. Dr. Mura says the test could mean knowing years sooner if a lung transplant might be needed.
“We developed a method that is actually quite inexpensive to increase the diagnostic accuracy of the biopsy and help to avoid unclassifiable cases. The method has a prognostic value, so it helps predict survival of these patients at the time of biopsy,” says Dr. Mura, who is also an Associate Professor of Medicine at Western University.
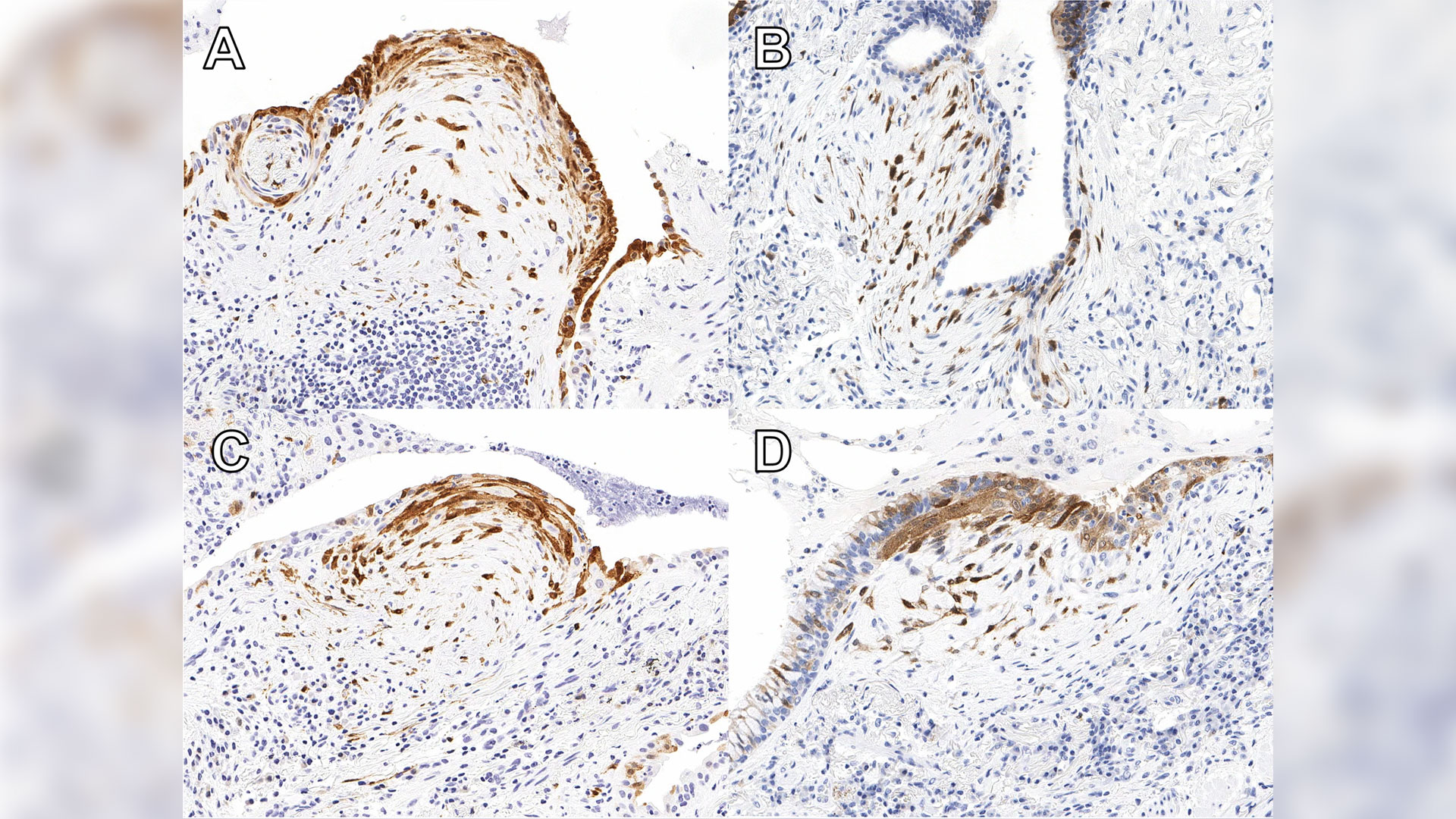
Patients with high expression of p16 were shown to experience worse outcomes than others with ILD, indicating that it is essential for these patients to start necessary treatment without delay. The protein is already widely used in ovarian cancer diagnosis. Now additional ongoing studies will help reinforce the value of the test for IPF diagnosis.
“We have no tests that we can apply to the (lung) biopsy other than the pathologist looking at it and saying ‘OK, this biopsy shows this pattern,’” Dr. Mura says. “There were absolutely zero additional biomarker tests to reinforce, validate or support the diagnosis. So, this will be the first time that we implement such test biomarkers in clinical practice.”
With this type of test that looks at the quantity of the biomarker, there is also the possibility of applying artificial intelligence to advance diagnosis in the future.
The research was supported by a 2018 Internal Research Fund grant from Lawson.
Lawson Health Research Institute is one of Canada’s top hospital-based research institutes, tackling the most pressing challenges in health care. As the research institute of London Health Sciences Centre and St. Joseph’s Health Care London, our innovation happens where care is delivered. Lawson research teams are at the leading-edge of science with the goal of improving health and the delivery of care for patients. Working in partnership with Western University, our researchers are encouraged to pursue their curiosity, collaborate often and share their discoveries widely. Research conducted through Lawson makes a difference in the lives of patients, families and communities around the world. To learn more, visit www.lawsonresearch.ca.
Communications Consultant & External Relations
Lawson Health Research Institute
T: 519-685-8500 ext. ext. 64059
C: 226-919-4748
@email
New device could reduce COVID-19 infection risk and demand for invasive ventilators
LONDON, ON – Led by Lawson Health Research Institute, London Health Sciences Centre (LHSC), University Health Network (UHN) and General Dynamics Land Systems-Canada (GDLS-Canada), researchers have designed a non-invasive ventilation mask that could significantly reduce aerosolization – the production of airborne respiratory droplets that may contain viruses or bacteria – when treating patients with COVID-19. The new device aims to reduce infection risks associated with non-invasive ventilation and lessen the demand for invasive ventilators. It is currently being tested through a clinical trial with patients at LHSC.
“Since the beginning of this pandemic, there have been global concerns about a shortage of ventilators,” says Dr. Tarek Loubani, Lawson Associate Scientist and Emergency Department Physician at LHSC. “Non-invasive ventilators like CPAP (continuous positive airway pressure) and BiPAP (bi-level positive airway pressure) machines are associated with an increased risk of COVID-19 transmission and so many hospitals have moved directly to invasive ventilation.”
COVID-19 is primarily spread through inhalation of respiratory droplets and the most severely ill patients require a ventilator to help them breathe. Unlike invasive ventilators, which require intubation, non-invasive ventilators help patients breathe through a mask that provides positive pressure to keep the lungs open and functioning. While non-invasive ventilators may be effective for some COVID-19 patients, their use comes with a much higher risk of spreading infection through aerosolization of respiratory droplets.
The team’s non-invasive ventilation mask aims to eliminate this risk. The novel device is customized from a standard firefighter’s mask using 3D printing and can be attached to any CPAP or BiPAP machine. Unlike traditional masks, it creates two tight seals – one around the patient’s nose and mouth and another around the face. Patients breathe in and out of a filter that captures any viral particles before they are released to the air.
“There are countless CPAP and BiPAP machines idling around the world while all resources go towards invasive ventilation,” explains Dr. Azad Mashari, Anesthesiologist at UHN’s Peter Munk Cardiac Centre. “Our mask aims to put these machines back into the clinician’s toolkit. By eliminating air leaks, we can improve patient safety and significantly reduce the risk of contracting COVID-19 for health-care workers and other patients.”
Drs. Loubani, Mashari and Benjamin Thomson, Nephrologist at Mackenzie Health, were part of a clinical research team that worked with engineers from GDLS-Canada to develop the device within six days.
“GDLS-Canada responded quickly to the urgent need to support those on the COVID-19 healthcare frontlines during this global health emergency,” says Doug Wilson-Hodge, GDLS-Canada’s Manager of Communications, Community and Government Relations. “The innovative design was very much a collaborative effort between all parties to contribute solutions to the COVID-19 pandemic.”
The initial clinical trial will test the device with up to 50 patients at LHSC’s Victoria Hospital and University Hospital with plans to expand to UHN. In addition to patients with COVID-19, participants will include those with asthma, chronic obstructive pulmonary disease (COPD) and congestive heart failure (CHF).
The research team anticipates other hospitals in Ontario and across Canada will join the study to create a multi-centre clinical trial. The device will be used in emergency departments and has potential to be used in intensive care units, remote nursing stations and during pre-hospital transport. It has also been designed for easy production in resource-strained locations.
“This problem affects everyone and it’s critical that we all do what we can to help,” adds Dr. Loubani. “We hope it will help not only those in urban centres like Toronto and London, but people in remote communities around the world.”
The trial is being supported with funding from Glia, an organization internationally recognized for producing medical supplies that are easily accessible and can be manufactured in low-resource settings.
-30-
DOWNLOADABLE MEDIA
Images




Video



Lawson Health Research Institute is one of Canada’s top hospital-based research institutes, tackling the most pressing challenges in health care. As the research institute of London Health Sciences Centre and St. Joseph’s Health Care London, our innovation happens where care is delivered. Lawson research teams are at the leading-edge of science with the goal of improving health and the delivery of care for patients. Working in partnership with Western University, our researchers are encouraged to pursue their curiosity, collaborate often and share their discoveries widely. Research conducted through Lawson makes a difference in the lives of patients, families and communities around the world. To learn more, visit www.lawsonresearch.ca.
London Health Sciences Centre has been at the forefront of medicine in Canada for 145 years and offers the broadest range of specialized clinical services in Ontario. Building on the traditions of its founding hospitals to provide compassionate care in an academic teaching setting, London Health Sciences Centre is home to Children’s Hospital, University Hospital, Victoria Hospital, the Kidney Care Centre, two family medical centres, and two research institutes – Children’s Health Research Institute and Lawson Health Research Institute. As a leader in medical discovery and health research, London Health Sciences Centre has a history of over 70 international and national firsts and attracts top clinicians and researchers from around the world. As a regional referral centre, London Health Sciences Centre cares for the most medically complex patients including critically injured adults and children in southwestern Ontario and beyond. The hospital’s nearly 15,000 staff, physicians, students and volunteers provide care for more than one million patient visits a year. For more information, visit www.lhsc.on.ca.
University Health Network consists of Toronto General, recently voted one of the Top 5 Hospitals in the World according to Newsweek Magazine, and Toronto Western Hospital, the Princess Margaret Cancer Centre, Toronto Rehabilitation Institute, and the Michener Institute of Education at UHN. The scope of research and complexity of cases at University Health Network has made it a national and international source of discovery, education and patient care. It has the largest hospital-based research program in Canada, with major research in cardiology, transplantation, neurosciences, oncology, surgical innovation, infectious diseases, genomic medicine and rehabilitation medicine. University Health Network is a research hospital affiliated with the University of Toronto. www.uhn.ca
Senior Media Relations Consultant
Communications & Public Engagement
T: 519-685-8500 ext. 73502
Celine.zadorsky@lhsc.on.ca
New imaging solution could help improve survival for patients with recurring prostate cancer
London, ON - A multicentre study led by London Health Sciences Centre Research Institute (LHSCRI), Lawson Research Institute of St. Joseph’s Health Care London (Lawson), and University Health Network (UHN) has found a novel imaging solution, called prostate-specific membrane antigen (PSMA) positron emission tomography (PET) scanning, can more effectively detect the recurrence of prostate cancer compared to standard imaging methods, and is associated with improved survival outcomes. The study, carried out over seven years, is published in The Journal of Nuclear Medicine.
During a PSMA PET scan, a radioactive molecule designed to target a protein in prostate cancer cells is injected into the bloodstream of a patient prior to the scan. The study uncovered that the molecule is effective in binding to prostate cancer cells, helping to detect recurring prostate cancer earlier and more effectively than standard imaging methods.
“This new technique gives physicians the information needed to determine the best treatment,” says Dr. Glenn Bauman, Scientist at LHSCRI and Radiation Oncologist at London Health Sciences Centre (LHSC). “When a blood test shows cancer has returned but standard imaging can’t find it, physicians may need to use less precise therapies like whole-body drug therapy. With this new imaging technique, we can locate the cancer and target it directly.”
The research team found that the overall detection rate was 70 per cent, much higher than the historical rates of detection of 10-20 per cent with conventional bone scan and CT scans. About half of all patients had their management of the disease changed based on the results of the scans. Almost 90 per cent of men with cancer detected by PSMA PET had a change in management of their recurring prostate cancer following the scan. They also found that patients who had their treatments modified based on results from the PET scan had a better overall survival rate than those who received standard imaging.
“We’re encouraged by how this imaging approach is already changing cancer care,” says Dr. Ur Metser, Division Head of Molecular Imaging at UHN and Clinician Scientist at UHN’s Princess Margaret Cancer Centre. “Our study showed that PET scans using this technique led to more personalized treatment decisions and those changes are linked to longer survival. That’s a meaningful step forward for patients and their care teams.”
Dr. Bauman and his colleagues from Lawson and LHSCRI were the first in Canada to image a patient using PSMA PET imaging in 2016. Since then, this study has enrolled thousands of men across six hospitals in Ontario through funding from Ontario Health - Cancer Care Ontario. Based on promising results from this and other research, PSMA PET scans are now funded as a standard of care test for men with advanced prostate cancer.
For more information, please contact: Deb Flaherty, Consultant, Communications & Public Affairs, St. Joseph's Health Care London.
519-646-6100 ext. 47560
ABOUT LAWSON RESEARCH INSTITUTE
Lawson Research Institute, the health innovation arm of St. Joseph's Health Care London, is committed to making and sharing discoveries that improve lives locally and internationally. Every day, Lawson researchers work to transform imagination to innovation to patient impact. Lawson leads health-care research. Find us at LawsonResearch.ca and @stjosephslondon on social media.
ABOUT LONDON HEALTH SCIENCES CENTRE RESEARCH INSTITUTE
At London Health Sciences Centre Research Institute (LHSCRI), our teams pioneer discoveries that transform the health of adult and paediatric patients around the world. As the research institute of London Health Sciences Centre (LHSC), we conduct research where patient care is delivered, working alongside patients, families, health-care providers and academic partners like Western University. We are leaders in advancing the understanding, diagnosis, treatment and management of diseases and health conditions through a diverse research program that ranges from laboratory-based science to clinical trials. Our research has a global impact as we build on LHSC’s 150-year legacy of health innovation and drive forward medical breakthroughs that
make a difference in the lives of patients and their families. Find us online at WWW.LHSCRI.CA and on social media @LHSCRI.
New imaging tool for diagnosing heart disease
An international team led by scientists from Lawson Health Research Institute and Cedars-Sinai Medical Center are the first to show that Magnetic Resonance Imaging (MRI) can be used to measure how the heart uses oxygen for both healthy patients and those with heart disease.
Reduced blood flow to the heart muscle is the leading cause of death in the Western world. Currently, the diagnostic tests available to measure blood flow to the heart require injection of radioactive chemicals or contrast agents that change the MRI signal and detect the presence of disease. There are small but finite associated risks and it is not recommended for a variety of patients including those with poor kidney function.
Standard methods
More than 500,000 of these tests are performed each year in Canada. A patient suspected of coronary heart disease for example may have reasonably normal blood flow at rest but as soon as they exercise they have pain or feel out of breath. They need more oxygen delivered to the heart tissue but due to vessels being compromised that doesn’t happen.
The standard technique is usually done in two days with the goal of seeing if the heart can increase blood flow when more oxygen is needed. The first test studies the patient at rest to see what the blood flow is like in the heart. This is a nuclear medicine imaging test that requires radioactive material to be injected and takes about an hour or more to complete.
They next day, they come for the same test but with the introduction of a stressor. That can be physical exercise but more often they are given an injection of a chemical drug which stimulates the heart and increases blood flow. This is in addition to a second injection of the radioactive material. The heart is imaged to see the level of oxygen getting to different parts of the heart and whether there are obstructions or reduction in size of the surrounding arteries.
A new stress test
“We wanted a non-invasive way to image the heart and replace the stress stimulus, and drastically reduce the amount of time needed for testing,” says Dr. Frank Prato, Lawson Assistant Director for Imaging. “This new method, cardiac functional MRI (cfMRI), does not require needles or chemicals being injected into the body. It eliminates the existing risks and can be used on all patients."
The team included researchers from Lawson; Cedars-Sinai Medical Center and University of California; King’s College in the United Kingdom; University Health Network and the University of Toronto; Siemens Healthineers; and, University of Edinburgh in the United Kingdom.
“Our discovery shows that we can use MRI to study heart muscle activity,” explains Dr. Prato. “We’ve been successful in using a pre-clinical model and now we are preparing to show this can be used to accurately detect heart disease in patients.”
To replace the stress test, this new technique uses repeat exposure to carbon dioxide to test how well the heart’s blood vessels are working to deliver oxygen to the muscle. A breathing machine changes the concentration of carbon dioxide in the blood. Levels are brought up for three minutes and then back down to normal four times. These changes should result in a change in blood flow to the heart, but does not happen when disease is present.
The cfMRI method reliably detects whether these changes are present and is comparable to the information gathered from the current two-day technique – in much less time and without injections. Dr. Prato notes that “we don’t want to stress the heart. We want to see whether there is capacity in the heart to increase blood flow if the heart needs to work harder.”
A brilliant discovery
Other researchers have explored oxygenation-sensitive MRI but initial results contained a high level of ‘noise’ with blurry images. Project leader and partner Dr. Rohan Dharmakumar, Associate Director of the Biomedical Imaging Research Institute at Cedars-Sinai Medical Center, believed that the noise was actually variation in the heart’s processing of oxygen. He engineered a way to average this variation and through testing at Lawson the team discovered that the noise is actually a new way to study how the heart works.
“We’ve opened the door to a new era and totally novel way of doing cardiac stress testing to identify patients with ischemic heart disease” says Dr. Dharmakumar. “This approach overcomes the limitations of all the current diagnostics – there would no longer be a need for injections or physical stress testing like running on treadmills.”
Through investigating this technique, they learned that the blurry images were showing normal physiological variability. People often think of heart rate as being stable, but in fact a heart that is unable to keep up with stressors indicates that disease is developing. In a healthy heart, the oxygen distribution to the tissue needs to vary.
“It’s a very exciting time. We had to bring all the technologies together to be able to image these kinds of changes in blood flow moment to moment,” says Dr. Prato.
He adds that “using MRI will not only be safer than present methods, but also provide more detailed information and much earlier on in the disease process.” Following initial testing through clinical trials, he sees this being used with patients clinically within a few years.
Moving forward
In addition to studying coronary artery disease, the method could be used in other cases where heart blood flow is affected such as the effects of a heart attack or damages to the heart during cancer treatment. Due to its minimal risk, this new tool could be safely used with the same patient multiple times to better select the right treatment and find out early on if it is working. Dr. Prato notes that “with this new window into how the heart works, we have a lot to explore when it comes to the role of oxygen in health and disease.”
The next steps of the research include a proof of principle study in London, Ontario with 20 patients. Following standard tests using the conventional technique at other sites, the participants will then come in for the experimental test to show that it produces the same result. The research would then move into a multi-centre clinical trial internationally.
The study “Accurate needle-free assessment of myocardial oxygenation for ischemic heart disease in canines using Magnetic Resonance Imaging” is published in Science Translational Medicine.
New national strategy to tackle dementia
Researchers in London, Ontario have been awarded $1.345 million over five years through the second phase of the Canadian Consortium on Neurodegeneration in Aging (CCNA), announced today as part of Canada’s first national dementia strategy. CCNA is a collaborative research program tackling the challenge of dementia and other neurodegenerative illnesses.
A Dementia Strategy for Canada: Together We Aspire focuses on preventing dementia, advancing therapies and finding a cure, as well as improving quality of life for people living with dementia and caregivers.
Clinician researchers from across the country working together
Dr. Manuel Montero-Odasso, Scientist at Lawson Health Research Institute, is world renowned for his findings on the relationship between cognition and mobility in the elderly, and gait as a predictor of frailty and dementia. He leads the Mobility, Exercise and Cognition (MEC) Team in London, comprised of top researchers in the areas of mobility, exercise and brain health.

“Evidence from other countries with dementia strategies shows that coordinated, targeted efforts at the national level improves results for all aspects of dementia care and also for research,” says Dr. Montero-Odasso, also a geriatrician and Director of the Gait and Brain Lab at Parkwood Institute, a part of St. Joseph’s Health Care London.
CCNA was purpose-built to synergize dementia research within the Canadian context. Phase I saw the creation of infrastructure fostering collaboration amongst Canadian researchers, and there are now 20 teams built around important research topics.
“This kind of effective national collaboration by scientists and clinicians from many disciplines gives the CCNA a cutting edge in research, prevention, treatment and management of all forms of dementia,” explains Dr. Montero-ODasso. “We created a national network of researchers form west to east coast with a high level of expertise to deliver lifestyle interventions to improve cognition and slow down progression to dementia. I feel privileged working with such excellent investigators and leading this important endeavour locally.”
Preventing dementia through lifestyle changes
The MEC team has several projects in the works, but the majority of the new funding is to complete the SYNERGIC Trial, SYNchronizing Exercises and Remedies on Gait and Cognition.
This first-in-the-world clinical study is testing a triple intervention aimed at treating Mild Cognitive Impairment (MCI) and delaying the onset of dementia. The SYNERGIC Trial incorporates physical exercises and cognitive training, along with vitamin D supplementation to determine the best treatment for improving mobility and cognition.
“We are looking at how interventions will work together and targeting cognitive decline at its earliest stage – individuals with MIC,” explains Dr. Montero-Odasso. “Both physical and cognitive exercises have shown promising effects for maintaining cognition, while vitamin D deficiency is associated with cognitive decline.”
A professor at Western University’s Schulich Medicine & Dentistry, Dr. Montero-Odasso partners with researchers from across the city including Dr. Rob Bartha, imaging scientist at Schulich Medicine & Dentistry and Robarts Research Institute at Western University, and Dr. Kevin Schoemaker who leads the Laboratory for Brain and Heart Health.
Study participants in the SYNERGIC Trial are asked to complete an individualized and progressive routine of exercises and cognitive training three times a week for six months, with one final assessment at 12 months. The main site for the study is Parkwood Institute with the physical exercises taking place at the Labatt Health Sciences Building on the Western campus.
To date, 138 research patients has been recruited across multiple sites in Canada.
One participant’s experience

One day, Peter Saracino saw an advertisement about a research study. They were looking for participants who were a minimum age of 60 and had minor cognitive impairment. He felt he fit the bill and he was interested in this kind of research.
“I have family members who suffered from forms of dementia and Parkinson’s Disease. I really understand how hard it hits and I liked that this study was about prevention,” explains Peter.
Going into it, Peter thought he was in pretty good shape. He has two dogs and walks them regularly. “But by going to the gym and doing the exercises and faster-paced walking, I realized that I wasn’t in as good shape as I thought. My diet was under control but I was still taking blood pressure medication. I didn’t have much energy.”
After 10 weeks in the study, he feels better than he has for over a decade. “I can garden for longer. I took two notches off my belt. I no longer take my blood pressure medication. I actually feel younger.”
He remembered that last year he slipped and fell four times, which was very unusual for him. Part of his cognitive impairment is that he had trouble with balance, and that has improved for him as well.
Peter feels that “this is exactly the kind of research that the government should be investing in – an ounce of prevention is worth a pound of cure. This kind of research leads to keeping people independent and healthier as they get older. People are happier. They feel like doing more. There is no downside to improving someone’s health through lifestyle changes, and in fact it is cost effective and helps ease the burden on the health care system.”
Looking forward
“Our preliminary analysis from SYNERGIC is giving us a strong indication that a multimodal approach, combining physical exercise, cognitive training and supplementation, has a synergistic effect. It seems the whole is greater than the sum of its parts,” says Dr. Montero-Odasso.
A major goal for the work of the MEC team in London is to translate their research findings into clinical guidelines that can be used at the front line of care. “Practitioners understand the overall importance of exercise and cognitive vitality, but we are missing more specific guidelines on what kind and how much will work for different patients. Basically, what is an effective lifestyle prescription.”

Dr. Montero-Odasso adds that “as our population ages, a comprehensive strategy is vital to ensure the growing number of those living with dementia receive the care and support they deserve. Over half a million Canadians are currently living with dementia. By 2031, this number is expected to nearly double.” More than one third of dementia cases might be preventable.
CCNA Phase II
In CCNA’s Phase II, researchers are working on analyzing the overall health of every patient in a large clinical cohort study, COMPASS-ND. This information will be used to enhance understanding of how changes in the brain affect dementia severity and ways to reduce and prevent this through lifestyle changes. Lawson is the leading recruitment site for COMPASS-ND and the London team will be instrumental in the larger lifestyle interventions moving forward.
CCNA is funded by the Government of Canada, Canadian Institutes of Health Research (CIHR) and other funding partners. CIHR is providing $31.6 million, and partners—including provincial agencies and non-profit organizations—are providing an additional $14.4 million for a total investment of $46 million over five years. The research on dementia prevention, diagnosis, treatment and care as part of Phase II of the CCNA will support the national strategy.