Minding the mysteries of Alzheimer's Disease
To researchers, Alzheimer’s Disease is mystifying. It has no known cause – and no cure.
But early detection can open the door to new hope. If someone knows that Alzheimer’s may be in their future, they could take part in clinical trials of new therapies to slow progression. And there are some disease-modifying treatments on the horizon, pending approval by Health Canada through clinical trial validation.
In London, access remains a major hurdle for patients. “We know there will be a lot of barriers to diagnosis and potential use of disease-modifying treatments because of current wait times to see a geriatrician,” says Sarah Best, Manager, Research Operations.
Now, a team at St. Joseph’s and Lawson Research Institute are pioneering a new local initiative to improve access to early diagnosis by testing for biomarkers when someone or their loved ones first notice memory difficulties. It’s made possible through generous donor support.
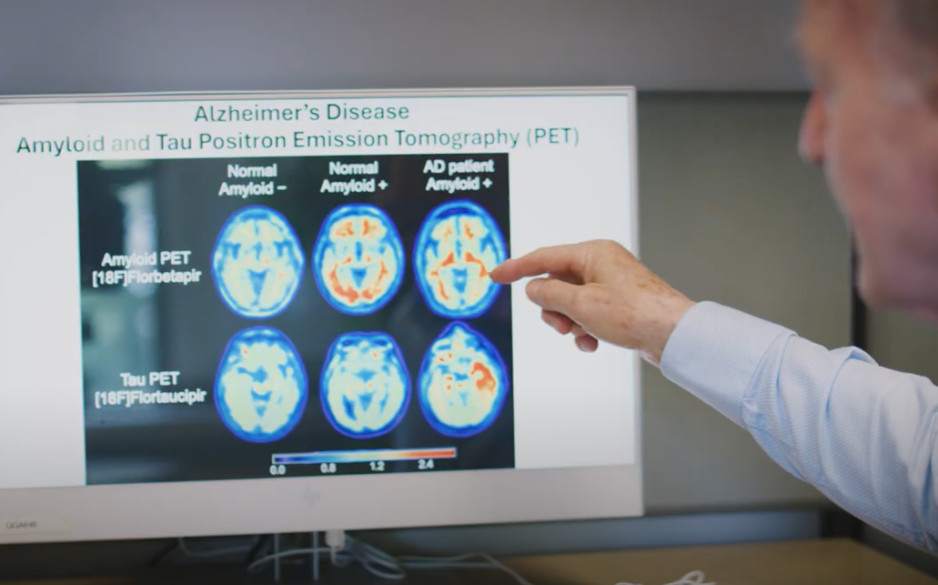
It’s known that a build-up of proteins like amyloid and tau in the brain is linked to Alzheimer’s Disease. Launched in 2024, the BioMIND study (Biomarkers for the Molecular Identification of Neurodegenerative Dementia) is exploring comprehensive testing to identify proteins and detect the disease in patients referred from St. Joseph’s Aging Brain and Memory Clinic.
Dr. Jaspreet Bhangu is leading this work with an impressive team that includes Dr. Michael Borrie, Dr. Jennie Wells, Dr. Ameya Patwardhan and Research Coordinator Kayla Vander Ploeg. The approach mirrors and adopts what is being done in other hospitals around the world.
“It can't be understated how exciting these breakthroughs are for patients suffering from memory concerns,” shares Dr. Bhangu. “Not only can we diagnose the disease more accurately, but we can also now give a more accurate prognosis and – most importantly – develop personalized treatment plans.”
So far, 100 people have taken part in BioMIND. To participate they need to be 55-85 years old and exhibit problems with memory that point to suspected cognitive impairment.
Participants undergo a lumbar puncture to analyze cerebrospinal fluid (CSF), have blood samples taken and are scanned with positron-emission tomography (PET) to image proteins in the brain. CSF is a clear liquid that protects the brain and spinal cord, and by analyzing it researchers can detect proteins and confirm someone’s PET results.
Taken together, this data paints a clear picture of the presence of Alzheimer’s Disease and could help predict how someone might respond to treatment. These biomarker tests identify the disease with over 90 per cent accuracy, years before traditional clinical methods can.
To date, seventy-five per cent of participants have tested positive for Alzheimer’s-related biomarkers. This pathway has significantly reduced their wait time to diagnosis and means they could take part in randomized controlled trials (RCTs) of disease-modifying therapies. RCTs are a necessary step before Health Canada can approve any new treatments.
“We were surprised at just how long the average wait times for these tests were,” says Bhangu. “Patients were waiting over a year, some almost 2 years, to be seen, receive all the testing and then receive results. By streamlining the testing, applying standardized criteria and dedicating resources to the problem, we were able to reduce that wait time to just over six months.”
The next steps for the research team are to continue gathering data, introduce diagnostic toolkits to better identify participants and form strategic partnerships with other hospitals in Southwestern Ontario. The goal is to one day move biomarker testing into real-world clinical settings – here in London and beyond.
For patients and their families, BioMIND could have lasting effects. “The ability to provide a more personalized treatment plan is powerful,” says Dr. Bhangu.
Interested in participating?
If you or a loved one are concerned about memory loss and are interested in learning more about research opportunities, contact the research team at: @email