Search
Search
Improving palliative cancer treatment with existing diagnostic scans: Study reveals promising results
A recent study from London Health Sciences Centre and Lawson Health Research Institute suggests that using existing diagnostic CT scans in planning simple palliative radiation treatments can significantly cut down the waiting time for urgent treatment, resulting in a better experience for cancer patients.
“Reducing the time patients spend in a cancer centre has far-reaching benefits,” said lead study author Melissa O’Neil an Advanced Practice Radiation Therapist at London Health Sciences Centre’s (LHSC) London Regional Cancer Program (LRCP). “Faster treatment initiation means quicker relief from symptoms for patients. Utilizing existing scans is also cost-effective and frees up appointment slots or staff, allowing us to accommodate and assist more patients in need.”

Palliative radiation therapy is used to relieve symptoms in patients whose cancers cannot be cured. It’s often used when tumours cause pain, neurological issues or breathing problems such as blocked airways.
In the current standard practice, patients referred for palliative radiation typically require a CT simulation scan before starting their treatment. This scan creates 3D images that the patient's health care team uses to develop a customized radiation treatment plan. Unfortunately, this process often takes several hours, even with efforts to speed it up.
However, many of these patients have undergone previous diagnostic CT scans as part of their routine medical care. Previous research has shown that radiation oncology teams can create suitable palliative treatment plans for patients with bone and soft tissue metastases using these existing scans. This approach is less time-consuming than the more intensive simulation scans.
In the current study, O’Neil and her colleagues explored whether using existing CT scans to plan treatment before a patient arrives at the cancer centre could reduce their wait time while still ensuring appropriate care. They randomly assigned 33 patients who needed palliative radiation for tumours in their chest, abdomen or pelvis to either the standard treatment planning with on-site CT simulation scans or to treatment planning using diagnostic CT scans taken within the previous 28 days.
The study found that patients who didn't need the extra CT simulation scan spent much less time at the cancer centre on the day of their treatment – just under 30 minutes compared to nearly five hours for the others. Treatments were delivered successfully, and patient perception on time spent at the cancer centre was improved for those whose treatment planning used diagnostic CT scans taken without the previous 28 days.
"For patients who need radiation to help treat symptoms of cancer, it's important for us to get them treated quickly and to minimize the time they spend waiting for medical appointments,” said Dr. David Palma, Radiation Oncologist at LHSC and Associate Scientist at Lawson. “This trial shows that this new approach not only saves resources by reducing the number of scans we do, but also substantially reduces the time patients spend waiting for urgent radiation.”
"These findings are incredibly promising, especially in light of the nationwide shortage of radiation therapists," said Dr. Michael Ott, Physician Department Executive for Oncology at LHSC. “Work like this has benefits that can reach far beyond London, offering more relief for patients across the country."
The findings were presented at the American Society for Radiation Oncology’s Annual Meeting on Oct. 3, 2023. This meeting is recognized globally as the leading radiation oncology scientific event, drawing more than 8,500 attendees each year.
While the study shows promise, the research team said it's important to note that using prior diagnostic scans may not be suitable for every type of cancer or patient. It depends on the specific area being treated and the technique used.
For more information, please contact:
Jessica Rabaey
Communications Consultant
London Health Sciences Centre
T: 519-685-8500 ext. 77728
Jessica.rabaey@lhsc.on.ca
Lawson Health Research Institute is one of Canada’s top hospital-based research institutes, tackling the most pressing challenges in health care. As the research institute of London Health Sciences Centre and St. Joseph’s Health Care London, our innovation happens where care is delivered. Lawson research teams are at the leading-edge of science with the goal of improving health and the delivery of care for patients. Working in partnership with Western University, our researchers are encouraged to pursue their curiosity, collaborate often and share their discoveries widely. Research conducted through Lawson makes a difference in the lives of patients, families and communities around the world. To learn more, visit www.lawsonresearch.ca.
Improving surgery for wrist arthritis
Wrist arthritis can cause debilitating pain, weakness and decreased range of motion. When patients are first diagnosed, the condition can often be managed with activity modification and pain medication. However, as symptoms progress, patients eventually require surgery.
Surgeons typically perform a procedure called four-corner fusion to preserve wrist motion and provide pain relief. This surgery involves removing one of the carpal bones and fusing four of the remaining carpal bones. Although this procedure is one of the most common treatments for wrist arthritis, it is not known how the position of the fusion of the wrist bones affects range of motion and joint contact.
Lawson associate scientist Dr. Nina Suh is leading a study with the goal of improving the surgical technique for four-corner fusion to maximize wrist function and symptom relief, and delay wrist arthritis progression.
Dr. Suh and her team will use a customized active-motion wrist simulator to create different carpal bone fusion positions. They will then assess how these positions affect wrist motion and joint contact area.
“We hope this research will lead to new surgical techniques that will help us to more effectively manage wrist arthritis with four-corner fusion,” says Dr. Suh, who is also an orthopaedic surgeon at the Roth McFarlane Hand and Upper Limb Centre (HULC) at St. Joseph’s Health Care London and an assistant professor at Western University’s Schulich School of Medicine & Dentistry. “The project will also advance our understanding of wrist biomechanics, providing a foundation for the development of enhanced patient-specific surgical tools, such as custom wrist fusion devices and implants.”
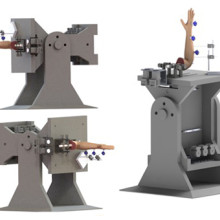
Image of the customized active-motion wrist simulator Dr. Nina Suh and her team are using to create different carpal bone fusion positions. They will then assess how these positions affect wrist motion and joint contact area.
The study is being funded through the Lawson Internal Research Fund (IRF), designed to allow scientists the opportunity to obtain start-up funds for new projects with exciting potential.
“The IRF program is valuable for scientists as external funding sources routinely require preliminary data to strengthen applications,” says Dr. Suh. “Particularly for new scientists such as myself, these grants provide seed funding that allows us to demonstrate the validity of our methodology and the clinical usefulness of our results.”
The IRF is designed to provide Lawson scientists the opportunity to obtain start-up funds for new projects with the potential to obtain larger funding, be published in a high-impact journal, or provide a clinical benefit to patients. Funding is provided by the clinical departments of London Health Sciences Centre and St. Joseph’s Health Care London, as well as the hospital foundations (London Health Sciences Foundation and St. Joseph’s Health Care Foundation).
Institute Team
Administration
Research Administration: responsible for the Lawson Approval process, delivery of Lawson’s Quality Assurance and Education program for clinical research, contract negotiation and approval for research, including industry-sponsored and investigator-initiated contracts, and fee-for-service support through Lawson Clinical Research Services.
Finance: responsible for the ongoing management of all research grants and contracts awarded to Lawson researchers; works closely with researchers, administrative staff, and funding sponsors to ensure adherence to funding guidelines and policies; manages the post-award functions for all research grants and contracts at the institute, including financial reporting, financial analysis and forecasting, cash flow and expenditure monitoring, compliance oversight, audit facilitation, and communicating with funding sponsors.
Research Human Resources: responsible for providing human resource services for Lawson researchers and staff, including acting as liaison between hospital Human Resources (HR) departments and Western faculties; the health and safety component facilitates standardizing safety processes and ensuring relevant legislation and safety standards are being met.
Research Operations & Technical Services: responsible for organizing the operations of Lawson’s vivarium/animal care facilities and services, as well as coordinating laboratory space, renovations, equipment and maintenance aspects of Lawson.
Research Informatics: responsible for supporting clinical researchers who have software development and database requirements by providing robust infrastructure to support their research activities, on a safe, secure IT platform to ensure patient confidentiality for clinical activities; and providing assistance with application development, data collection, data extraction, archiving, collaboration, analysis and reporting.
Strategic Planning and Development
Communications & External Relations: responsible for building and managing the Lawson brand and reputation, including public relations, media relations, marketing, special events, web presence and social media, advocacy, strategic planning and issues management.
Grant Development: responsible for facilitating the full spectrum of research grant submissions, including grant coordination for large government grant applications; development of grantsmanship; dissemination of new information pertaining to research and training grants opportunities; and, institution submissions and sign-off processes for CIHR, CFI, ORF, etc.
Business Development: responsible for providing services to Lawson investigators to facilitate the transfer of medical research from the laboratory to commercial use, including assisting with patenting new discoveries and finding commercial partners for collaborative research and licensing. Expertise is offered in the areas of intellectual property protection, marketing, licensing agreements and formation of start-up companies. Commercialization opportunities at Lawson are managed through WORLDiscoveries®, the business development arm of London’s extensive research network and the bridge between local invention and global industry.
Research Infrastructure: responsible for the development and implementation of a research master plan for all Lawson sites, including identifying potential research space solutions to meet the evolving needs of researchers, and working with the Facilities Planning departments at both hospitals to operationalize research space plans.
Institutional Research Data Management Strategy
Table of Contents
3 Research Data and Importance of Research Data Management. 2
6.1 Awareness-Raising Activities. 4
6.3 Promote and Support RDM Practices. 5
6.4 Access to RDM Tools, Resources, and Infrastructure. 5
6.4.1 Information Technology (IT) Infrastructure. 5
9 Indigenous Data Considerations. 8
10 Other Relevant Strategies and Policies. 9
10.1 London Health Sciences Centre Corporate Policies and Procedures. 9
10.2 St. Joseph’s Health Care London Policies and Procedures. 10
10.3 External Strategies/Policies. 10
11 Acronyms & Abbreviations. 12
Introduction
The federal research funding agencies (Tri-Agency: Canadian Institutes of Health Research (CIHR), Natural Sciences and Engineering Research Council of Canada (NSERC), Social Sciences and Humanities Research Council (SSHRC)) released a Research Data Management Policy in March 2021. They have set a deadline for March 1, 2023, for each postsecondary institution and research hospital eligible to administer CIHR, NSERC, or SSHRC funds to develop an institutional strategy for Research Data Management (RDM), and notify the agencies when it has been completed. Additionally, for specific funding opportunities, the agencies require data management plans (DMPs) to be submitted to the appropriate agency at the time of application. Furthermore, grant recipients are required to deposit into a digital repository all digital research data, metadata, and code that directly support the research conclusions in journal publications and pre-prints that arise from agency-supported research.
Lawson Research Institute is the research arm of St. Joseph’s Health Care London. Lawson recognizes the impact of funding on research, the RDM requirements and obligations implemented by funding agencies, and the importance of research data management. Lawson is fully engaged in developing and implementing the Institutional RDM Strategy.
The Tri-agency is committed to funding research that is conducted to the highest professional and disciplinary standards, is performed ethically, makes effective use of public funds, is verifiable and replicable, and that makes results as accessible as possible. The agencies support the FAIR (Findable, Accessible, Interoperable, and Reusable) guiding principles and accordingly advocate an increased ability for research data to be archived, found, and responsibly reused to fuel discoveries and innovation across multiple disciplines and geographical borders. Research data management is, therefore, a necessary component of achieving research excellence.
Research data is the data used as evidence to support and validate research findings or results and used as input for analysis. Research data is derived from source data. This can include information extracted from original sources such as clinical systems, experiments, simulations, etc. It is to be noted that any data containing identifiable personal information must remain private and confidential.
Research Data Management is the organization and maintenance of research data throughout the entire research project lifecycle. This includes setting up protocols before initiating data collection, and then collecting, tracking, and creating backups of the data during study execution, and eventually, data sharing, archiving, and publishing upon project completion. This is not a new concept. In fact, Lawson researchers have been employing these processes and procedures and performing RDM in varying capacities. However, with the new policy requirements, obligations for regulatory compliance, concerns for privacy and security, initiatives for data sharing and reproducibility, a push for the FAIR principles, the open science movement, and a need to elevate the availability of Canadian data on the world stage, it is imperative for Lawson to implement and support RDM best practices and procedures.
The Lawson institutional RDM strategy is a concise and directive document that outlines how Lawson will increase its capacity to support and foster a culture of effective research data management. This institutional strategy is a collaboration between internal and external key stakeholders. It will support Lawson researchers in managing their data throughout the research lifecycle using appropriate data stewardship and data management practices.
This strategy applies to all research data generated and collected by Lawson researchers, research trainees, and research staff, whether the research was funded by the Tri-agency or other funders, or self-funded.
This strategy does not propose the creation of new or amendment of existing hospital policies.
The RDM Advisory Committee supports establishing and implementing the overall institutional strategy. Drafting of the institutional RDM strategy is being led by the RDM Project Team, comprising Research Informatics, Grants Development, Quality Assurance, and Research Administration team members through Lawson and (jointly with London Health Sciences Centre Research Institute) through a shared Office of Research Services
The RDM Advisory Committee, comprising key institutional stakeholders, acts as a resource to the RDM Project Team on the planning, implementation, and ongoing evaluation of the strategy. These stakeholders include Lawson’s Chief Operating Officer, representatives from the hospital Privacy Offices, Information Technology Services, LHSC Data Governance, Research Ethics Boards, Western Libraries, Lawson Approvals, Grants Development, Quality Assurance, Research Directors, research teams, and research trainees. The RDM Project Team consults with other stakeholders and community partners as needed to support the RDM rights of all stakeholders involved in research.
The Advisory Committee further understands that as the research landscape advances, the RDM requirements and obligations implemented by Tri-agency and other funders may change; as RDM progress is made as outlined in this strategy, the resources and priorities will also change, necessitating re-evaluation of RDM maturity and revision of this RDM strategy. Hence, the strategy will be considered a living document that will be reviewed on an annual basis by the Advisory Committee.
Lawson aims to meet RDM requirements and implement RDM best practices and processes to fully support its researchers and research communities. Through the implementation of the institutional RDM strategy, Lawson will provide sustainable support and solutions by documenting existing support and processes, formalizing responsibilities, and expressing and promoting RDM best practices. Lawson aims to support researchers in establishing and implementing data management practices consistent with ethical, legal, and commercial obligations.
6.1 Awareness-Raising Activities
The Lawson research community was engaged through various means to raise awareness about the Tri-Agency RDM policy requirements and RDM.
Data champions were recruited from different departments to help promote the value of RDM and engage with various communities.
The research community was invited to participate in an anonymous survey to understand the current state of Lawson in developing and allocating human, organizational, infrastructure, and financial resources for Research Data Management within the Lawson research community.
The RDM Maturity Assessment, based on Maturity Assessment Model in Canada (MAMIC), was conducted to take stock of the current services and identify areas for future growth and development. It helped to capture the perceptions of the current RDM service offerings.
Lawson also established an internal RDM website to provide information about research data management, Tri-Agency RDM Policy requirements, data management plans, institutional RDM strategy, frequently asked questions, and resources. This website provides up-to-date information and keeps the research community informed.
The RDM Project team hosted webinars on research data management, data management plan templates, and DMP Assistant.
The objective is to assist the broader research community in understanding the institution’s current and planned RDM capacity, challenges, and needs. Therefore, to facilitate an ongoing dialogue and collaboration on the advancement of RDM on a national level, Lawson has created web pages dedicated to RDM on an external-facing website.
Lawson has research data management expertise and skills within different departments. However, a centralized and integrated approach to support RDM is required. Infrastructure support for large data sets can also generate some human resources issues. New research data management approaches also require the upkeep of skills, techniques, processes, and solutions. Accordingly, appropriate knowledge and skill development will be needed for the team to support RDM.
6.3 Promote and Support RDM Practices
Lawson will continue to support researchers and their staff by encouraging a data management culture and an environment that promotes and facilitates research data management. Lawson’s Research Informatics and Grants Development teams will provide Data Management Plans and data deposit consultation services.
6.4 Access to RDM Tools, Resources, and Infrastructure
6.4.1 Information Technology (IT) Infrastructure
Lawson’s IT infrastructure is managed and supported by the hospitals’ Information Technology Services (ITS) department.
ITS provides access to OneDrive, Teams, SharePoint, Webex, Office 365, MS Project, and many more solutions and platforms available for research purposes.
All systems available to Lawson researchers through ITS are backed up on a nightly and monthly basis. Backups on tapes are also stored at an off-site location for disaster recovery.
Lawson-supported servers are hosted at the secure ITS data centre. These servers are configured and secured as per hospital guidelines.
Various safeguards have been implemented and documented to prevent, detect, and mitigate the effects of computer viruses, worms, or other potentially harmful software code on study data and software.
The virtual servers hosted at the hospitals’ data centre employ vMotion. It allows IT to move running virtual machines from one physical server to another without impacting end-users. vMotion keeps the IT environment up and running, providing unprecedented flexibility and availability. It also decreases downtime and improves reliability by supporting business continuity and disaster recovery procedures.
Lawson researchers have access to many support services through different departments.
The RDM Project team hosts workshops related to RDM and DMPs.
The Research Informatics team hosts workshops to build capacity in researchers to accelerate the build of high-quality data capture projects.
Quality Assurance and Education Program hosts ‘Lunch ‘n Learn’ sessions related to policies and regulations, and educates researchers and staff on compliance requirements and best practices.
ITS has partnered with Microsoft and peer Ontario hospitals to develop Live Virtual Training sessions to allow learning at an individual pace.
Several tools are available to Lawson researchers to support their RDM requirements.
REDCap is a Research Electronic Data Capture web-based tool for creating and managing online database applications and surveys. Hosted at the hospitals’ data centre, Lawson Research Informatics administrates this secure platform to meet the diverse research needs of the Lawson community.
Lawson supports its researchers by providing robust infrastructure to support their research activities on a safe, secure IT platform to ensure patient confidentiality for clinical activities; and by providing hosting for customized web applications for research studies. Lawson manages several research applications on Windows and Linux servers, securely hosted at the London hospitals’ data centre.
File Safe and M365 are the recommended tools available to Lawson researchers to securely transfer files, large and small, including confidential or patient-identifiable information, instead of using email attachments.
DMP Assistant is a national, online, bilingual data management planning tool to assist researchers in preparing data management plans (DMPs). Lawson researchers and their team members can create an account on this platform. They can select ‘Lawson Health Research Institute’ as their organization to develop their discipline or study-specific Data Management Plan through a series of critical data management questions supported by best-practice guidance and examples.
Lawson researchers also have access to the Lawson DMP Template that guides them through all the elements of a data management plan and provides example answers to several questions.
Canadian Repository Options
Borealis, a publicly accessible, secure Canadian data repository system managed by Western Libraries, allows for data to be released and shared openly or privately with precision at the file level using Dataverse software. This is available to Lawson researchers who are faculty members at Western University.
Lawson researchers can utilize the national Federated Research Data Repository (FRDR) platform to deposit data or to search for and download data across Canadian repositories. This platform can efficiently ingest datasets of any size, and preservation processing is done automatically. Research data can be ingested, curated, preserved, discovered, cited, and shared from this single platform.
International Repository Options
The Directory of Open Access Repositories (OpenDOAR) provides a quality-assured list of open-access repositories worldwide. OpenDOAR staff harvest and assign metadata to allow categorization and analysis to assist the wider use and exploitation of repositories. OpenDOAR is based at the University of Nottingham.
Re3data.org is a global registry of research data repositories that covers research data repositories from different academic disciplines. It includes repositories for the permanent storage and access of data sets to researchers, funding bodies, publishers, and scholarly institutions. Re3data.org promotes a culture of sharing, increased access, and better visibility of research data.
Several key stakeholders were identified internally and externally from the organization. The RDM Advisory Committee was formed in March 2022 to include relevant stakeholders who are directly impacted by the implementation of the Institutional Strategy. This committee included stakeholders from Executive Administration, Research Informatics, Grants Development, and representatives from London Health Sciences Centre and St. Joseph’s Privacy Offices, Information Technology Services, LHSC Data Governance, Western University’s Health Sciences Research Ethics Board, Western Libraries, Lawson Approvals, Quality Assurance, Research Directors, Research teams, and a Postdoctoral Fellow. The Advisory Committee meets monthly to help raise awareness, assess institutional readiness, and serve as a communication medium. Appropriate delivery mechanisms for outreach were implemented to engage the Lawson research community. The input and feedback from the research community were solicited through surveys, webinars, online RDM sites, email, ad hoc meetings, etc.
Perceiving the significance of collaboration with external stakeholders and community partners, the RDM Project Team has been reaching out to Indigenous Cancer Care Unit Clinical Institutes & Quality Programs and the Office of Inclusion & Social Accountability (Indigenous Health) at LHSC, and the Knowledge Exchange, Impact & EDI-D in Research office and Indigenous Health Lab at Western University for finding a common intersection of work and continued consultation and consideration concerning RDM training and processes.
Lawson Research Institute supports researchers in adopting and complying with ethical, legal, and commercial obligations through various means. Research oversight and compliance are overseen by Western University’s Health Sciences Research Ethics Board (HSREB), Clinical Trials Ontario (CTO), and Ontario Cancer Research Ethics Board (OCREB). They also oversee the ethical conduct of research studies involving human participants. Additionally, the Tri-Agency’s Tri-Council Policy Statement: Ethical Conduct Involving Humans – TCPS2 (2018) provides guidance to researchers conducting research involving human participants.
Lawson’s Quality Assurance and Education Program (QAEP), which is a part of Lawson’s Quality Management System (QMS), facilitates research compliance across the organization through Standard Operating Procedures, Guidance Documents, Lunch and Learns, Clinical Research Training, Quality Assurance Reviews as well as providing research support to Investigators, research teams and other stakeholders on the regulations, policies and best practices governing clinical research.
Research compliance with legal and commercial obligations falls under the Lawson Research Approval Systems (Contracts) and WORLDiscoveries, a joint business development arm for Lawson, Western University, and Robarts Research Institute. Lawson’s Contracts team is responsible for drafting, reviewing, negotiating, and coordinating all contracts for research under Lawson’s auspices.
Lawson intends to support researchers involved with Indigenous research and ensure that Tri-Agency RDM policy requirements are addressed. We recognize that there are many Indigenous communities, peoples, cultures, languages, and protocols and therefore no singular approach can be applied. We also acknowledge the validity of Indigenous epistemologies and ontologies.
Lawson recognizes, supports, and respects Indigenous data sovereignty and their right to own, control, access, possess, and protect the information collected from these communities, based on free, prior, and informed consent. We are committed to respect and adhere to nation and community specific protocols by following research data management principles developed and approved by these communities, collectives and organizations such as the First Nations Information Governance Centre’s OCAP (Ownership, Control, Access, Possession) principles, the Inuit Tapiriit Kanatami National Inuit Strategy on Research, and Global Indigenous Data Alliance’s CARE principles. These govern data collection, ownership, protection, use and sharing to encourage inclusive development and innovation, and equitable outcomes. Lawson will ensure that the DMPs are co-developed with these communities, collectives, and organizations, in line with RDM principles and DMP formats that they accept. We acknowledge that they have the right to repatriate the data and this could result in exceptions to the data deposit requirement.
Lawson researchers are also guided through TCPS 2 (2018) – Chapter 9: Research Involving the First Nations, Inuit and Métis Peoples of Canada to ensure that research involving Indigenous peoples is postulated on respectful relationships that encourage collaboration and engagement between researchers and participants. It is a policy that serves as a framework for the ethical conduct of research involving Indigenous peoples in Canada.
It is our institutional responsibility to build capacity for doing this work in an effective way. Lawson is working with the Indigenous Cancer Care Unit Clinical Institutes & Quality Programs and the Office of Inclusion & Social Accountability (Indigenous Health) at LHSC. Lawson is also collaborating with the Office of Equity, Diversity, and Inclusion (EDI) and the Indigenous Health Lab at Western University.
Lawson aims to strengthen Indigenous research capacity by facilitating and promoting equitable access and support for Indigenous students and researchers. Lawson Scientists usually have Western faculty appointments and/or have employment as clinicians with St. Joseph’s. Lawson supports Indigenous researchers with these affiliations and encourages non-Indigenous researchers to co-develop new models for Indigenous research and research training with Indigenous communities. This may include co-developing research questions, agendas, respectful relations, and impactful solutions built on trust, respect, and mutual interests. Indigenous researchers and leadership will help non-Indigenous researchers understand Indigenous perspectives, needs, concerns and aspirations for Indigenous research. Indigenous researchers can help foster positive collaboration with Indigenous partners, collect organic responses from Indigenous participants and provide an Indigenous research lens on the collected information and analyzed data.
These efforts will help us create research data management guidelines for our researchers involved in Indigenous research, assuring the best practices reflect the Four R’s - Respect, Relevance, Reciprocity, and Responsibility.
Lawson Research Institute is governed by St. Joseph’s policies, processes, and procedures that are relevant to various aspects of RDM.
The Lawson RDM Strategy is also intended to align with external requirements and guidance, including provincial, federal, and international laws.
The pertinent policies and documents that were reviewed are listed below.
10.1 London Health Sciences Centre Corporate Policies and Procedures
Acceptable Use of Information Technology Resources |
Breach of Privacy |
Privacy |
Confidentiality |
Electronic Mail (Email) Use |
Acceptable Use of Information Technology Resources |
Records Retention and Disposition |
Remote Access to Computer Network Resources |
Security of Confidential Information and Information Technology Systems |
Use of Cellular Phones and Other Wireless Devices |
Use of Personal Health Information for Research, Education, and Quality Improvement |
Invention - Lawson |
Lawson Approval for Clinical Research |
10.2 St. Joseph’s Health Care London Policies and Procedures
Acceptable Use of Information Technology Resources |
Access and Disclosure of Personal Health Information |
Breach of Patient Privacy |
Clinical Trials Involving Investigational Drugs |
Confidentiality |
Disclosure of Patient Information, Samples, and/or Belongings to Law Enforcement Agents |
Electronic Mail (Email) Use |
Freedom of Information and Protection of Privacy Act (FIPPA) |
Health Record Management |
Interpretation and Translation Services |
Patient Requests to Restrict the Use and Disclosure of Personal Health Information |
Privacy |
Records Retention and Destruction |
Remote Access to Computer Network Resources |
Security of Confidential Information and Information Technology Systems |
Use of Personal Health Information for Research, Education, and Quality Assurance |
10.3 External Strategies/Policies
Acronym/Term | Definition |
CARE | Collective Benefit, Authority to Control, Responsibility, and Ethics |
CIHR | Canadian Institutes of Health Research |
DMP | Data Management Plan |
EDI | Equity, Diversity, and Inclusion |
FAIR | Findable, Accessible, Interoperable, and Reusable |
HSREB | Health Sciences Research Ethics Board |
ITS | Information Technology Services |
Lawson | Lawson Research Institute of St. Joseph's Health Care London |
LHSC and LHSCRI | London Health Sciences Centre and its research arm, London Health Sciences Centre Research Institute |
MAMIC | Maturity Assessment Model in Canada |
NSERC | Natural Sciences and Engineering Research Council of Canada |
OCAP | Ownership, Control, Access, and Possession |
OCREB | Ontario Cancer Research Ethics Board |
QAEP | Quality Assurance and Education Program |
QMS | Quality Management System |
RDM | Research Data Management |
SSHRC | Social Sciences and Humanities Research Council |
St. Joseph’s | St. Joseph’s Health Care London |
Tri-Agency | CIHR, NSERC, SSHRC |
WORLDiscoveries | WORLDiscoveries is the commercialization arm of Western University, Robarts, and Lawson and represents our commitment to protecting and transferring technologies developed by our partners to market. |
The definitions below are as per the Tri-Agency Research Data Management Policy, Frequently Asked Questions, and Social Sciences and Humanities Research Council Definition of Terms, 2021, Government of Canada.
Data Deposit
“Data deposit” refers to when the research data collected as part of a research project is transferred to a research data repository. The repository should have easily accessible policies describing deposit and user licenses, access control, preservation procedures, storage and backup practices, and sustainability and succession plans. The deposit of research data into appropriate repositories supports ongoing data-retention and, where appropriate, access to the data.
Data Management Plan
A data management plan (DMP) is a living document, typically associated with an individual research project or program that consists of the practices, processes, and strategies that pertain to a set of specified topics related to data management and curation. DMPs should be modified throughout a research project to reflect changes in project design, methods, or other considerations.
Indigenous Research
Research in any field or discipline that is conducted by, grounded in or engaged with First Nations, Inuit, Métis or other Indigenous nations, communities, societies or individuals, and their wisdom, cultures, experiences or knowledge systems, as expressed in their dynamic forms, past and present. Indigenous research can embrace the intellectual, physical, emotional and/or spiritual dimensions of knowledge in creative and interconnected relationships with people, places and the natural environment.
Metadata
“Metadata” are data about data—data that define and describe the characteristics of other data. Accurate and relevant metadata are essential for making research data findable. A principle to help determine what information should be included in metadata is the open archival information system model criterion that the information be “independently understandable.”
Research Data
Research data are data that are used as primary sources to support technical or scientific enquiry, research, scholarship, or creative practice, and that are used as evidence in the research process and/or are commonly accepted in the research community as necessary to validate research findings and results. Research data may be experimental data, observational data, operational data, third party data, public sector data, monitoring data, processed data, or repurposed data. What is considered relevant research data is often highly contextual, and determining what counts as such should be guided by disciplinary norms.
Research Data Management
Research data management (RDM) refers to the processes applied through the lifecycle of a research project to guide the collection, documentation, storage, sharing, and preservation of research data.
The Lawson Research Data Management Strategy is a living document that will be reviewed and shared on an annual basis. It will be revised and updated as the institutional and researchers’ research data management requirements, practices, and understanding advance.
In the coming years, Lawson will:
evaluate the changes in policy requirements,
assess evolving institutional RDM training needs
gauge availability of the supporting resources
ensure compliance with the RDM policies and requirements by funders, publishers, and legislative bodies
evaluate technological infrastructure and meet expanding storage requirements – this may include assessment of access on platforms like Borealis for Lawson researchers who are not Western faculty members
facilitate capacity-building events for RDM and DMP development
provide training and educational services and support
examine DMP evaluation criteria and guidelines
consider requirements for data deposit certifications
adjudge implications on the involved stakeholders
consult with community stakeholders to confirm Indigenous data sovereignty considerations
It has been clear that implementation of the institutional RDM strategy requires collaboration on a broader scale. To that end, the RDM Project Team will build additional meaningful connections and identify subject matter experts for future RDM projects. Furthermore, the RDM project team is also proposing to establish a Community of Practice to manage RDM requirements in a sustainable method. This Community of Practice may comprise key stakeholders and experts from groups such as Ethics, Privacy, QA, Indigenous wellness group, Office of Inclusion and Social Accountability, research investigators, research coordinators, and research personnel.
Interdisciplinary team explores London’s unique approach to addressing homelessness
In London, Ont. more than 1,700 people are experiencing homelessness.
Recognizing the growing crisis, more than 200 individuals from 70 organizations across the city came together and developed the Health & Homelessness Whole of Community System Response – a strategic roadmap for supporting unhoused people who have the most complex health and social support challenges, and helping them access permanent housing that meets their needs. Now a team of researchers has been tasked with assessing how well the roadmap is working.
To understand if the program is meeting its ambitious goals and explore areas for improvement, the Centre for Research on Health Equity and Social Inclusion (CRHESI), a community-university partnership based at Western’s Faculty of Health Sciences, was asked by the Whole of Community Response leaders to support and coordinate research and evaluation of the program.
CRHESI works with and reports to the System Foundation Table, a team of volunteer community partners from diverse sectors with a mandate to evaluate new activities and embed continuous improvement to ensure sustainability of the program.
“London’s Whole of Community Response is a novel approach to addressing the needs of the most vulnerable individuals experiencing complex housing and health challenges within our community,” said Mick Kunze, chair of the System Foundation Table. “While our approaches and interventions are rooted in evidence-based practices, we also understand that local context matters.”
This research and evaluation work is funded jointly by Western, London Health Sciences Centre and through a generous donation from local businessman Ryan Finch to St. Joseph’s Health Care Foundation. The three groups came together recognizing there is a need to create meaningful and measurable change in our community.
Their support has allowed CRHESI to recruit two research managers, Eleanor Gebrou and Kelly Barnes, who oversee the work of more than 100 researchers and community partners from organizations across the city participating in the evaluation project.
"Community-led initiatives not only reflect the real needs of individuals facing homelessness, but they also empower frontline workers by fostering an environment of collaboration and understanding. Together, we can create solutions that prioritize well-being for all—those we serve and those who serve," said Gebrou.
This work is split into four main research areas:
- Exploring the outcomes and experiences of people who are precariously housed, unhoused or at risk of homelessness, as well as the perspectives of the broader residents of London, Ont. and especially the business community.
- Evaluating the experiences and well-being of those working in jobs that provide care and housing support.
- Answering questions related to systems, structures, processes and costs of care.
- Evaluating the processes that enabled this large and complex “whole of community” response, and how it unfolds from here.
“I really believe in evidence-based decision making and having as much information as we can to go into program and policy design,” said Barnes. “It's so important to understand what parts of our homelessness response are working well and what can be improved. Research and evaluation are some of the best ways to do that.”
The importance of voices and stories
Research teams will collect quantitative data, including statistics on how many people get housed, how this housing affects the number of hospital and police contacts and the cost-benefit of these new activities. They’ll also collect qualitative data, which involves hearing the experiences of those without homes, those accessing care and support and those providing and leading services.
This evaluation framework will be presented to city council, sitting as the Strategic Priorities and Policies Committee, on October 8, 2024.
“It is really important to us that this not just be numbers,” said Barnes. “We know how important people’s voices and stories are. When we take the numbers and combine them with people’s descriptions of their experiences, and the discussion of their journey, that gives us really solid information to bring back to the policy-makers and program developers to show what’s working and what isn’t.”
This research and evaluation work will span the next two years, with results shared annually with city council starting in July 2025. In addition to annual reporting, research project teams will share data as it emerges.
“The research and evaluation efforts led by the backbone team of this movement, including the System Foundations Table and our CHRESI research and evaluation managers, will be instrumental in guiding our present and future work, by illustrating the real impact our interventions are having on the health and well-being of those we’re aiming to support, as well as by responding to the questions and concerns voiced by all members of our community,” said Kunze.
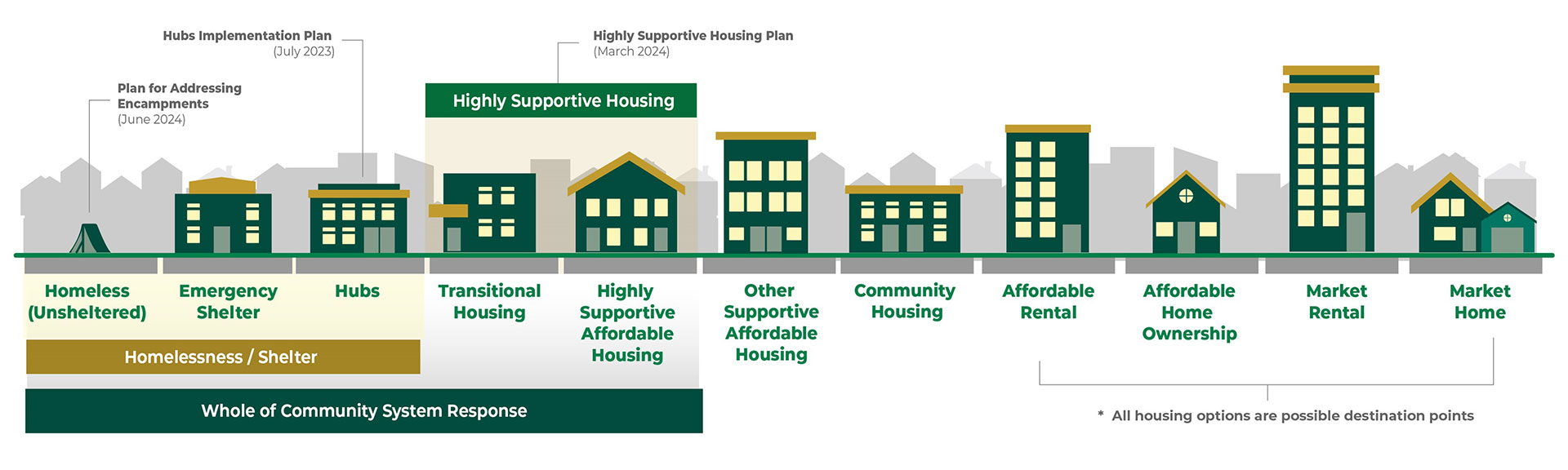
Health & Homelessness Whole of Community System Response
The Whole of Community Response is about developing wrap-around health care and housing supports for those who need them most, ensuring basic human needs are met and building trusting relationships with people so they’re set up to succeed. It includes three main pillars: establishing hubs to meet immediate needs for safe shelter, nutrition and hygiene, and allowing care providers to start the process of stabilizing people’s mental and physical health. Alongside establishing hubs, the broader plan includes bringing more highly supportive housing units to London and implementing a human rights-based approach to support people wherever they are on the housing spectrum.
City council endorsed the Whole of Community Response approach in March 2023. Since then, two hubs have been established, as well as 93 highly supportive housing units, with 50 more units in development, toward a goal of 600 highly supportive housing units within three years.
The Whole of Community Response is being facilitated through the City of London and implemented by lead agencies, with ongoing collaboration among several sectors, including police and emergency services, hospitals, front-line community service organizations, educational institutions and government.
The Health and Homelessness Fund for Change, fuelled by a transformative $25-million donation from a London, Ont. family who wishes to remain anonymous, primarily provides capital funding to help fast-track the creation of hubs and highly supportive housing units. The Fund for Change is administered by London Community Foundation in partnership with the donor family.
International Women's Day 2020
International Women’s Day, taking place on March 8, 2020, is a day to celebrate the various achievements of women around the world and is a call to action for gender parity. This year’s theme is “Each for Equal” to emphasize that each individual has a significant part in working towards a gender equal world.
This year, Lawson is highlighting the different paths for women who are working towards a career in the health sciences. Below are the perspectives from students, physicians and researchers on their contributions to science and medicine.

High school student, Nimrit Aulakh, is completing her co-op placement with Lawson Scientist, Dr. Cheryl Forchuk. Her research focuses on improving mental health care for youth.
“Science has always been of interest to me and has now become significant within my academic endeavours. Part of my desire to become involved in the sciences stemmed from my older sister, who exposed me initially to the research side of science. It is with the help of rationale and logic in science that I can enrich my academic experience. Specifically, I have been working as a co-op student alongside the Mental Health Nursing Research Alliance and learning what it means to be a researcher. During my time, I conducted preliminary analyses on one of their studies, which focuses on improving mental health care for youth through virtual models of care. I will be presenting my findings at the Thames Valley Science and Engineering Fair later this month and if successful, will advance to the Canada Wide Science Fair. This experience has shown me a new side of science, one that I hope that I can continue to be a part of. I realized that through science and research, I can contribute to advancing society. To continue my journey through post-secondary education, I have applied to get my Bachelor’s in Health Sciences. Both within and beyond my four years of undergraduate studies, I hope to continue my contributions in serving the public through scientific research, as well as create an image for girls with the same interests as me, everywhere.”

Romaisa Pervez is a Research Assistant working with Lawson Scientist, Dr. Arlene MacDougall. She recently completed her Master of Science in Epidemiology and Biostatistics at Western University.
“I’m the student lead on a project that’s titled “Building a Sustainable Model and Evaluation for Psychological Rehabilitation in Kenya: An Implementation Study.” I’m working with the CREATE (Community Recovery Achieved Through Entrepreneurism) Kenya team to conduct a study to improve how we deliver, evaluate, and train persons with lived experience or community members to facilitate the Psychosocial Rehabilitation (PSR) Toolkit so it can be locally sustained in Kenya. I’ve travelled to Kenya twice during my Master’s to build relationships with stakeholders and conduct focus groups/interviews. My passion lies in understanding how we can implement upstream initiatives for mental health that are both sustainable and effective. Furthermore, I want to explore how we can find leverage points within the current mental health system in low to middle income countries and create innovative solutions. In the near future, I want to pursue medicine and further my knowledge and build stronger skills in this field.”

Dr. Kelly Anderson is an Associate Scientist at Children’s Health Research Institute, a program of Lawson. She is also Assistant Professor in the Department of Epidemiology & Biostatistics at the Schulich School of Medicine & Dentistry, Western University.
“I lead a research program in public mental health research, with a primary focus on young people experiencing a first onset of psychotic disorder. Together with my team, we are investigating the distribution and risk factors for psychotic disorders, prevention in early psychosis, and access to care and utilization of services in first-episode psychosis. My research program is centered around mentorship and training of students from all levels, and I work with trainees to foster high-level skills in the design and analysis of epidemiologic studies using large complex datasets. I am committed to fostering a culture of equity, diversity, and inclusion within my research team, and I advocate strongly for gender and early career representation for awards, scientific symposia, and other career opportunities. As a female scientist, I regularly mentor young women, both formally and informally, and due to the focus of my research, I also regularly work with trainees with lived experience of mental disorders. The diverse experiences and perspectives of these students both inspire and inform our work together.”

Dr. Michelle Barton-Forbes is an Associate Scientist at Lawson and a Physician at London Health Sciences Centre (LHSC) specializing in paediatric infectious diseases. She is also an Assistant Professor at the Schulich School of Medicine & Dentistry, Western University.
“My research program is focused on the clinical epidemiology of infectious diseases in children, particularly in vulnerable paediatric populations such as neonates and young infants. A secondary area of interest is in bacterial resistance and antimicrobial stewardship. Through engagement in multicentre research and contribution to national guideline development, I am able to make a difference in the management and prevention of common childhood infections nationally. The combination of intense passion for my subspecialty, inquisitive curiosity and a drive to better understand common childhood illnesses is infectious to my students. I motivate my students to excellence by inspiring them to believe in themselves and their ability to make a difference. My students are encouraged and challenged to find answers to unanswered questions and unexplained trends through research. As a proud Canadian and an immigrant from a nation that prides itself in its diversity, I believe that diversity is our strength. The Jamaican national motto “out of many one people” has framed my worldview and has influenced my practice.”

Dr. Eileen Crowley is a Scientist at Lawson and a Pediatric Gastroenterologist at LHSC. She is also an Assistant Professor at the Schulich School of Medicine & Dentistry, Western University.
“My research interests include pediatric inflammatory bowel disease (IBD), the genetics of IBD, big datasets, therapeutic drug monitoring clinical trial endpoints and precision medicine. My work has served to be better delineate the genetic phenotype of children with IBD as well as optimizing response to therapy in this age group. I engage students and motivate them to work with enthusiasm! My aim is to create learning opportunities that are active, collaborative and promote learning relationships. Once I have identified a student’s goal, it is easier to share and attain that goal. Within the research setting, I aim to maintain an environment where every student feels accepted, valued and safe. Sharing of ideas creates opportunities whilst also fostering a sense of personal belonging and achievement.”
To learn more about International Women’s Day, visit https://www.internationalwomensday.com/.
Investing in life-changing research
Through donor support, endowed research chairs are exploring and answering some of the most profound and complex research questions of our time.
Among cherished family photos and special mementos in the office of Jeremy Burton, PhD, is a slightly faded photo of a young woman. Burton points out the framed photo as he enthusiastically talks about his work. It’s a young Miriam Burnett, after whom the Miriam Burnett Chair in Urological Sciences is named. It’s also the first endowed research chair position Burton held at St. Joseph’s Health Care London (St. Joseph’s).
As the research chair for seven years, Burton speaks fondly about the relationship he has with the Burnett family and the crucial role their support has played in advancing his research.
“Thanks to their funding, we became one of the world leaders in urological microbiome research,” he says.
Endowed research chairs at St. Joseph’s receive consistent and sustainable funding so that research leaders and their teams can answer the most profound and complex health questions of our time.
For decades, donors have been inspired by the clinical research taking place at St. Joseph’s and have heavily invested in endowed research chairs. Today, St. Joseph’s Health Care Foundation manages seven endowed chairs focused on several areas, including molecular imaging, fetal and newborn growth and diabetes. Working in partnership with Western University, and with donor support, the foundation recently established four new endowed chairs in mobility, medical biophysics, medical imaging and ophthalmology.
“Medical research in Canada is chronically underfunded, and there is almost no sustainable funding for hospital-based research positions,” says Michelle Campbell, President & CEO, St. Joseph’s Health Care Foundation. “Private philanthropy has filled that gap for years. When a donor gives to an endowed research chair, they are building capacity in the present day and creating future value and opportunity. An endowed gift has a multiplier effect.”
Burton, now the endowed Research Chair in Human Microbiome and Probiotics, has many reasons to be grateful for this support. Not only does the endowed fund pay for Burton’s research salary, it also partially supports the salaries of a lab manager and technical team – all vital for a sophisticated lab to be successful.
The funding also provides the gift of time – a diminishing commodity for any busy research team.
“Scientists need more time to think,” says Burton, a Lawson Research Institute (Lawson) scientist. “We are incrementally being stretched in multiple directions, and the funding gives us the time to do what we are meant to do – find answers to important clinical questions and find solutions to medical problems.”
Distinguished Lawson scientist and university professor Cheryl Forchuk, PhD, wholeheartedly agrees. She recently completed her final term as The Beryl and Richard Ivey Research Chair in Aging, Mental Health, Rehabilitation & Recovery, another endowed position. As Chair, Forchuk provided scientific and administrative leadership to a large group of researchers based at St. Joseph’s Parkwood Institute focused on mental health, activity and mobility, and cognitive vitality and brain health.
Many research leaders, she explains, can afford to spend only two days a week on their own research projects. Endowed chair positions change that.
“Imagine travelling across the country to create a national study focused on homelessness, two days a week at a time,” she suggests candidly. “You couldn’t.”
Forchuk is referring to her landmark project to better understand how many people in Canada are homeless and who they are. The goal was to develop more accurate sources of data and recommend appropriate support and services. Her work is already resulting in important changes.
Today, Forchuk is embarking on another cross-country research project to find solutions related to homelessness for Canadian veterans who are women.
Like Forchuk, Burton’s Chair position requires him to provide operational and research leadership, including developing research networks and partnerships nationally and internationally to advance studies that will revolutionize care.
“As the Chair, I think it is important that I have wide-ranging projects that benefit people in our own community and beyond,” says Burton, who is optimistic about the outcomes of several of his team’s studies.
He recently partnered with London’s First Episode Mood and Anxiety Program to study the impact of fermented foods on the microbiome of young people taking medications for mental health conditions.
One of the side effects of these medications is weight gain, which deters some patients from taking it. By providing patients with slow-release apple cider capsules, which have similar properties to fermented foods and positively affect the microbiome, they have seen an overall improvement in participants’ mental health and cholesterol after just a few months.
Reflecting on his team’s research achievements to date and the potential of what’s to come, Burton emphasizes how vital endowed chairs are to the sustainability of research and the hope to translate newly discovered knowledge into medical practice.
“Research funding from other sources comes and goes,” he says, “but endowed chair positions that are focused on improving human health provide continuity, build research and create change benefiting all of us.”
Jamie Fleet
Jamie Fleet, MD
Assistant Professor, Schulich School of Medicine and Dentistry
Stroke
Dr. Jamie Fleet is a physiatrist at Parkwood Institute and an Assistant Professor in the Department of Physical Medicine and Rehabilitation at the Schulich School of Medicine and Dentistry at Western University. Dr. Fleet completed medical school as well as residency training in Physical Medicine and Rehabilitation at McMaster University. She is currently enrolled in a Master’s program in Clinical Epidemiology through the Health Research Methodology program at McMaster University with a focus on fracture treatment and prevention in older patients after stroke. Her primary clinical area of focus is in stroke rehabilitation.
Though still early in her career, Dr. Fleet has developed a strong research background, primarily focusing on drug safety studies in older adults using large data through ICES. Her other research interests include exercise and health promotion/prevention strategies in patients after stroke, as well as fall prevention strategies and pain management. "

Jenny Thain
Jenny Thain, MD
Assistant Professor, Schulich School of Medicine and Dentistry
Balance, Gait and Falls; Implementation Science and Education
Dr. Jenny Thain is a geriatrician at Parkwood Institute and an Assistant Professor within the Division of Geriatric Medicine at the Schulich School of Medicine and Dentistry at Western University. Dr. Thain completed a Master’s degree in Health Professions Education through the University of Maastricht and completed her medical training at the University of Nottingham, sub-specializing in orthogeriatric medicine.
Her areas of clinical interest include osteoporosis and bone health, with a particular interest in orthogeriatric care. She is the Chair of the Canadian Geriatrics Society Osteoporosis and Bone Health Special Interest Group, a member of the Osteoporosis Canada Scientific Advisory Council and Fragility Fracture Network Scientific Committee and the Geriatric clinical lead of the Hip Fracture Unit at Victoria Hospital, London.
